Lennard-Jones model: Difference between revisions
Carl McBride (talk | contribs) |
|||
| (109 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
== Lennard-Jones potential = | The '''Lennard-Jones''' [[intermolecular pair potential]] is a special case of the [[Mie potential]] and takes its name from [[ Sir John Edward Lennard-Jones KBE, FRS | Sir John Edward Lennard-Jones]] | ||
<ref>[http://dx.doi.org/10.1098/rspa.1924.0081 John Edward Lennard-Jones "On the Determination of Molecular Fields. I. From the Variation of the Viscosity of a Gas with Temperature", Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character '''106''' pp. 441-462 (1924)] § 8 (ii)</ref> <ref>[http://dx.doi.org/10.1098/rspa.1924.0082 John Edward Lennard-Jones "On the Determination of Molecular Fields. II. From the Equation of State of a Gas", Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character '''106''' pp. 463-477 (1924)] Eq. 2.05</ref>. | |||
The Lennard-Jones [[models |model]] consists of two 'parts'; a steep repulsive term, and | |||
smoother attractive term, representing the London dispersion forces <ref>[http://dx.doi.org/10.1007/BF01421741 F. London "Zur Theorie und Systematik der Molekularkräfte", Zeitschrift für Physik A Hadrons and Nuclei '''63''' pp. 245-279 (1930)]</ref>. The Lennard-Jones potential is probably the most extensively studied model potential today <ref name="Stephan2019">[https://doi.org/10.1021/acs.jcim.9b00620 Simon Stephan, Monika Thol, Jadran Vrabec, and Hans Hasse "Thermophysical Properties of the Lennard-Jones Fluid: Database and Data Assessment", Journal of Chemical Information and Modeling '''59, 10''' pp. 4248–4265 (2019)]</ref>. Apart from being an important model in itself, | |||
the Lennard-Jones potential frequently forms one of 'building blocks' of many [[force fields]] and molecular-based equation of state models <ref name="Stephan">[https://doi.org/10.1016/j.fluid.2020.112772 Simon Stephan, Jens Staubach, Hans Hasse "Review and comparison of equations of state for the Lennard-Jones fluid", Fluid Phase Equilibria '''523''' pp. 112772 (2020)]</ref>. It is worth mentioning that the 12-6 Lennard-Jones model is not the | |||
most faithful representation of the potential energy surface, but rather its use is widespread due to its computational expediency. | |||
For example, the repulsive term is maybe better described with the [[exp-6 potential]]. | |||
One of the first [[Computer simulation techniques |computer simulations]] using the Lennard-Jones model was undertaken by Wood and Parker in 1957 <ref>[http://dx.doi.org/10.1063/1.1743822 W. W. Wood and F. R. Parker "Monte Carlo Equation of State of Molecules Interacting with the Lennard‐Jones Potential. I. A Supercritical Isotherm at about Twice the Critical Temperature", Journal of Chemical Physics '''27''' pp. 720- (1957)]</ref> in a study of liquid [[argon]]. Thermophysical property data sampled by molecular simulations since then until 2019 has been compiled, digitalized and assessed by Stephan et al <ref name="Stephan2019">[https://doi.org/10.1021/acs.jcim.9b00620 Simon Stephan, Monika Thol, Jadran Vrabec, and Hans Hasse "Thermophysical Properties of the Lennard-Jones Fluid: Database and Data Assessment", Journal of Chemical Information and Modeling '''59, 10''' pp. 4248–4265 (2019)]</ref>. This database contains about 35,000 datapoints. | |||
The | == Functional form == | ||
The Lennard-Jones potential is given by | |||
:<math> | :<math> \Phi_{12}(r) = 4 \epsilon \left[ \left(\frac{\sigma}{r} \right)^{12}- \left( \frac{\sigma}{r}\right)^6 \right] </math> | ||
or is sometimes expressed as | |||
:<math> \Phi_{12}(r) = \frac{A}{r^{12}}- \frac{B}{r^6}</math> | |||
* <math> \sigma </math> : | where | ||
* <math>r := |\mathbf{r}_1 - \mathbf{r}_2|</math> | |||
* <math> \ | * <math> \Phi_{12}(r) </math> is the [[intermolecular pair potential]] between two particles or ''sites'' | ||
* <math> \sigma </math> is the value of <math>r</math> at which <math> \Phi_{12}(r)=0</math> | |||
* <math> \epsilon </math> is the well depth (energy) | |||
* <math>A= 4\epsilon \sigma^{12}</math>, <math>B= 4\epsilon \sigma^{6}</math> | |||
* Minimum value of <math> \Phi_{12}(r) </math> at <math> r = r_{min} </math>; | |||
: <math> \frac{r_{min}}{\sigma} = 2^{1/6} \simeq 1.12246 ... </math> | |||
In reduced units: | |||
* Density: <math> \rho^* := \rho \sigma^3 </math> | |||
where <math> \rho := N/V </math> (number of particles <math> N </math> divided by the volume <math> V </math>) | |||
* Temperature: <math> T^* := k_B T/\epsilon </math> | |||
where <math> T </math> is the absolute [[temperature]] and <math> k_B </math> is the [[Boltzmann constant]] | |||
The following is a plot of the Lennard-Jones model for the Rowley, Nicholson and Parsonage parameter set <ref>[http://dx.doi.org/10.1016/0021-9991(75)90042-X L. A. Rowley, D. Nicholson and N. G. Parsonage "Monte Carlo grand canonical ensemble calculation in a gas-liquid transition region for 12-6 Argon", Journal of Computational Physics '''17''' pp. 401-414 (1975)]</ref> (<math>\epsilon/k_B = </math> 119.8 K and <math>\sigma=</math> 0.3405 nm). See [[argon]] for other parameter sets.<br> | |||
[[Image:Lennard-Jones.png|500px]] | |||
==Critical point== | |||
The location of the [[Critical points |critical point]] for the untruncated potential has been assessed to be at <ref name="Stephan2019">[https://doi.org/10.1021/acs.jcim.9b00620 Simon Stephan, Monika Thol, Jadran Vrabec, and Hans Hasse "Thermophysical Properties of the Lennard-Jones Fluid: Database and Data Assessment", Journal of Chemical Information and Modeling '''59, 10''' pp. 4248–4265 (2019)]</ref> | |||
:<math>T_c^* = 1.321 \pm 0.007 </math> | |||
at a reduced density of | |||
:<math>\rho_c^* = 0.316 \pm 0.005 </math> | |||
and the critical [[pressure]] of | |||
:<math>p_c^* = 0.129 \pm 0.005. </math> | |||
The critical [[compressibility factor]] is given by <ref>[http://dx.doi.org/10.1063/1.4829837 V. L. Kulinskii "The critical compressibility factor of fluids from the global isomorphism approach", Journal of Chemical Physics '''139''' 184119 (2013)]</ref> | |||
:<math>Z_c = \frac{p_cv_c}{RT_c} = 0.281</math> | |||
Vliegenthart and Lekkerkerker | |||
<ref>[http://dx.doi.org/10.1063/1.481106 G. A. Vliegenthart and H. N. W. Lekkerkerker "Predicting the gas–liquid critical point from the second virial coefficient", Journal of Chemical Physics '''112''' pp. 5364-5369 (2000)]</ref> | |||
<ref>[http://dx.doi.org/10.1063/1.3496468 L. A. Bulavin and V. L. Kulinskii "Generalized principle of corresponding states and the scale invariant mean-field approach", Journal of Chemical Physics ''''133''' 134101 (2010)]</ref> | |||
have suggested that the critical point is related to the [[second virial coefficient]] via the expression | |||
:<math>B_2 \vert_{T=T_c}= -\pi \sigma^3</math> | |||
====Truncated at <math>2.5 \sigma</math>==== | |||
For the potential truncated at <math>2.5 \sigma</math> <ref>[http://dx.doi.org/10.1063/1.4944926 Ernesto S. Loscar, C. Gastón Ferrara1, and Tomás S. Grigera "Spinodals and critical point using short-time dynamics for a simple model of liquid", Journal of Chemical Physics '''144''' 134501 (2016)]</ref> | |||
:<math>T_c^* = 1.1875 (15)</math> | |||
:<math>p_c^* = 0.1105 (15)</math> | |||
==Triple point== | |||
The location of the [[triple point]] as found by Mastny and de Pablo <ref name="Mastny"> [http://dx.doi.org/10.1063/1.2753149 Ethan A. Mastny and Juan J. de Pablo "Melting line of the Lennard-Jones system, infinite size, and full potential", Journal of Chemical Physics '''127''' 104504 (2007)]</ref> is | |||
:<math>T_{tp}^* = 0.694</math> | |||
:<math>\rho_{tp}^* = 0.84</math> (liquid); | |||
:<math>\rho_{tp}^* = 0.96</math> (solid). | |||
==Radial distribution function== | |||
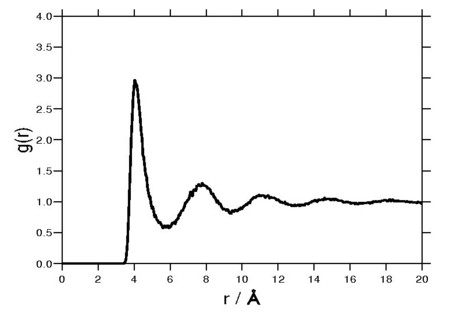
The following plot is of a typical [[radial distribution function]] for the monatomic Lennard-Jones liquid<ref>[http://dx.doi.org/10.1063/1.1700653 John G. Kirkwood, Victor A. Lewinson, and Berni J. Alder "Radial Distribution Functions and the Equation of State of Fluids Composed of Molecules Interacting According to the Lennard-Jones Potential", Journal of Chemical Physics '''20''' pp. 929- (1952)]</ref> (here with <math>\sigma=3.73</math>Å and <math>\epsilon=0.294</math> kcal/mol at a [[temperature]] of 111.06K): | |||
[[Image:LJ_rdf.png|center|450px|Typical radial distribution function for the monatomic Lennard-Jones liquid.]] | |||
==Helmholtz energy function== | |||
An expression for the [[Helmholtz energy function]] of the [[Building up a face centered cubic lattice | face centred cubic]] solid has been given by van der Hoef <ref>[http://dx.doi.org/10.1063/1.1314342 Martin A. van der Hoef "Free energy of the Lennard-Jones solid", Journal of Chemical Physics '''113''' pp. 8142-8148 (2000)]</ref>, applicable within the density range <math>0.94 \le \rho^* \le 1.20</math> and the temperature range <math>0.1 \le T^* \le 2.0</math>. For the liquid state see the work of Johnson, Zollweg and Gubbins <ref>[http://dx.doi.org/10.1080/00268979300100411 J. Karl Johnson, John A. Zollweg and Keith E. Gubbins "The Lennard-Jones equation of state revisited", Molecular Physics '''78''' pp. 591-618 (1993)]</ref>. | |||
==Vapor-liquid equilibrium== | |||
The vapor-liquid equilibrium of the Lennard-Jones potential has been studied more than 45 times in the literature. Several of these data sets were found to have gross deviations to an entity of data sets, which is both thermodynamically consistent and in good mutual agreement <ref name="Stephan2019">[https://doi.org/10.1021/acs.jcim.9b00620 Simon Stephan, Monika Thol, Jadran Vrabec, and Hans Hasse "Thermophysical Properties of the Lennard-Jones Fluid: Database and Data Assessment", Journal of Chemical Information and Modeling '''59, 10''' pp. 4248–4265 (2019)]</ref>. The mutual agreement of these data sets was found to be approximately <math>\pm 1%</math> for the vapor pressure, <math>\pm 0.75%</math> for the enthalpy of vaporization, <math>\pm 0.2%</math> for the saturated liquid density, and <math>\pm 1%</math> for the saturated vapor density <ref name="Stephan2019">[https://doi.org/10.1021/acs.jcim.9b00620 Simon Stephan, Monika Thol, Jadran Vrabec, and Hans Hasse "Thermophysical Properties of the Lennard-Jones Fluid: Database and Data Assessment", Journal of Chemical Information and Modeling '''59, 10''' pp. 4248–4265 (2019)]</ref>. | |||
:<math>\ln p^s = n_1 T + \frac{n_2}{T} + \frac{n_3}{T^{n_4}} ~~ \mathrm{with} ~~ n_i =[ 1.156551, -4.431519, -0.423028, 2.638743 ] </math> | |||
:<math>\Big(\frac{\rho'}{\rho_\mathrm{c}}\Big) = 1 + \sum_{i=1}^{5} n_i\, \Big(1 - \frac{T}{T_\mathrm{c}} \Big)^{t_i} ~~ \mathrm{with}~~ n_i = [1.3417, 2.075332, -2.123475, 0.328998, 1.386131] ~~\& ~~ t_i = [0.32714, 0.958759, 1.645654, 17.000001, 2.400858] </math> | |||
:<math>\ln \Big(\frac{\rho''}{\rho_\mathrm{c}}\Big) = \sum_{i=1}^{5} n_i\, \Big(1 - \frac{T}{T_\mathrm{c}} \Big)^{t_i} ~~ \mathrm{with}~~ n_i = [-8.135822, -102.91911, -3.037979, -44.381841, -34.55892948] ~~\& ~~ t_i = [1.651685, 43.469214, 0.462877, 11.500462, 5.39437] </math> | |||
:<math>\Delta h_v = \sum_{i=1}^{4} n_i\, (T_\mathrm{c} - T)^{t_i} ~~ \mathrm{with} ~~ n_i = [6.456728, 2.700099, -3.073573, 3.149052 ] ~~\& ~~ t_i = [0.411342, 0.460416, 2.350953, 5.01701 ] </math> | |||
==Melting line== | |||
The solid and liquid densities along the [[Melting curve |melting line]] <ref>[http://dx.doi.org/10.1063/1.4937487 D. M. Heyes and A. C. Brańka "The Lennard-Jones melting line and isomorphism", Journal of Chemical Physics '''143''' 234504 (2015)]</ref> are given by the following equations: | |||
====van der Hoef==== | |||
van der Hoef (Ref. <ref>[http://dx.doi.org/10.1063/1.1314342 Martin A. van der Hoef "Free energy of the Lennard-Jones solid", Journal of Chemical Physics '''113''' pp. 8142-8148 (2000)]</ref> Eqs. 25 and 26): | |||
:<math>\rho_{\mathrm {solid}} = \beta^{-1/4} \left[ 0.92302 - 0.09218 \beta + 0.62381 \beta^2 -0.82672 \beta^3 + 0.49124 \beta^4 -0.10847 \beta^5\right]</math> | |||
and | |||
:<math>\rho_{\mathrm {liquid}} = \beta^{-1/4} \left[ 0.91070 - 0.25124 \beta + 0.85861 \beta^2 -1.08918 \beta^3 + 0.63932 \beta^4 -0.14433 \beta^5\right]</math> | |||
====Mastny and de Pablo==== | |||
Mastny and de Pablo (Ref <ref>[http://dx.doi.org/10.1063/1.2753149 Ethan A. Mastny and Juan J. de Pablo "Melting line of the Lennard-Jones system, infinite size, and full potential", Journal of Chemical Physics '''127''' 104504 (2007)]</ref> Eqs. 20 and 21): | |||
:<math>\rho_{\mathrm {solid}} = \beta^{-1/4} \left[ 0.908629 - 0.041510 \beta + 0.514632 \beta^2 -0.708590\beta^3 + 0.428351 \beta^4 -0.095229 \beta^5\right]</math> | |||
and | |||
:<math>\rho_{\mathrm {liquid}} = \beta^{-1/4} \left[ 0.90735 - 0.27120 \beta + 0.91784 \beta^2 -1.16270\beta^3 + 0.68012 \beta^4 -0.15284 \beta^5\right]</math> | |||
A study has been performed of the solid-fluid equilibria, and behavior in the high density region <ref>[http://dx.doi.org/10.1063/1.4990667 Andreas Köster, Peter Mausbach, and Jadran Vrabec "Premelting, solid-fluid equilibria, and thermodynamic properties in the high density region based on the Lennard-Jones potential", Journal of Chemical Physics '''147''' 144502 (2017)]</ref>. | |||
==Zeno line== | |||
It has been shown that the Lennard-Jones model has a straight [[Zeno line]] <ref>[http://dx.doi.org/10.1021/jp802999z E. M. Apfelbaum, V. S. Vorob’ev and G. A. Martynov "Regarding the Theory of the Zeno Line", Journal of Physical Chemistry A '''112''' pp. 6042-6044 (2008)]</ref> on the [[Phase diagrams: Density-temperature plane |density-temperature plane]]. | |||
==Widom line== | |||
It has been shown that the Lennard-Jones model has a [[Widom line]] <ref>[http://dx.doi.org/10.1021/jp2039898 V. V. Brazhkin, Yu. D. Fomin, A. G. Lyapin, V. N. Ryzhov, and E. N. Tsiok "Widom Line for the Liquid–Gas Transition in Lennard-Jones System", Journal of Physical Chemistry B Article ASAP (2011)]</ref> on the [[Phase diagrams: Pressure-temperature plane | pressure-temperature plane]]. | |||
==Viscosity== | |||
[[Viscosity]] <ref>[https://doi.org/10.1063/1.5018483 Kai-Min Tu,  Kang Kim, and  Nobuyuki Matubayasi "Spatial-decomposition analysis of viscosity with application to Lennard-Jones fluid", Journal of Chemical Physics '''148''' 094501 (2018)]</ref>. | |||
==Perturbation theory== | |||
The Lennard-Jones model is also used in [[Perturbation theory |perturbation theories]], for example see: [[Weeks-Chandler-Andersen perturbation theory]]. | |||
== Approximations in simulation: truncation and shifting == | == Approximations in simulation: truncation and shifting == | ||
The Lennard-Jones model is often used with a cutoff radius of <math>2.5 \sigma</math>, beyond which <math> \Phi_{12}(r)</math> is set to zero. Setting the well depth <math> \epsilon </math> to be 1 in the potential on arrives at <math> \Phi_{12}(r)\simeq -0.0163</math>, i.e. at this distance the potential is at less than 2% of the well depth. For an analysis of the effect of this cutoff on the melting line see the work of Mastny and de Pablo <ref name="Mastny"></ref> and of Ahmed and Sadus <ref>[http://dx.doi.org/10.1063/1.3481102 Alauddin Ahmed and Richard J. Sadus "Effect of potential truncations and shifts on the solid-liquid phase coexistence of Lennard-Jones fluids", Journal of Chemical Physics '''133''' 124515 (2010)]</ref>. See Panagiotopoulos for critical parameters <ref>[http://dx.doi.org/10.1007/BF01458815 A. Z. Panagiotopoulos "Molecular simulation of phase coexistence: Finite-size effects and determination of critical parameters for two- and three-dimensional Lennard-Jones fluids", International Journal of Thermophysics '''15''' pp. 1057-1072 (1994)]</ref>. See Pártay for the ground state structure <ref>[http://dx.doi.org/10.1039/C7CP02923C Lívia B. Pártay, Christoph Ortner, Albert P. Bartók, Chris J. Pickard and Gábor Csányi "Polytypism in the ground state structure of the Lennard-Jonesium", Physical Chemistry Chemical Physics '''19''' 19369 (2017)]</ref>. It has recently been suggested that a truncated and shifted force cutoff of <math>1.5 \sigma</math> can be used under certain conditions <ref>[http://dx.doi.org/10.1063/1.3558787 Søren Toxvaerd and Jeppe C. Dyre "Communication: Shifted forces in molecular dynamics", Journal of Chemical Physics '''134''' 081102 (2011)]</ref>. In order to avoid any discontinuity, a piecewise continuous version, known as the [[modified Lennard-Jones model]], was developed. | |||
== | == Cutoff Lennard-Jones potential== | ||
The cutoff Lennard-Jones potential is given by (Eq. 2 in <ref>[http://dx.doi.org/10.1103/PhysRevA.8.1504 Spotswood D. Stoddard and Joseph Ford "Numerical Experiments on the Stochastic Behavior of a Lennard-Jones Gas System", Physical Review A '''8''' pp. 1504-1512 (1973)]</ref>): | |||
:<math> \Phi_{12}(r) = 4 \epsilon \left\{ \left[ \left(\frac{\sigma}{r} \right)^{12}- \left( \frac{\sigma}{r}\right)^6 \right]+ \left[ 6\left(\frac{\sigma}{r_c} \right)^{12}- 3\left( \frac{\sigma}{r_c}\right)^6 \right] \left(\frac{r}{r_c} \right)^2 -7 \left(\frac{\sigma}{r_c} \right)^{12} + 4 \left(\frac{\sigma}{r_c} \right)^{6} \right\}</math> | |||
: <math> | where <math>r_c</math> is the cutoff radius. | ||
== n-m Lennard-Jones potential == | |||
It is relatively common to encounter potential functions given by: | |||
: <math> \Phi_{12}(r) = c_{n,m} \epsilon \left[ \left( \frac{ \sigma }{r } \right)^n - \left( \frac{\sigma}{r} \right)^m | |||
\right]. | \right]. | ||
</math> | </math> | ||
with <math> n </math> and <math> m </math> being positive integers and <math> n > m </math>. | |||
<math> c_{n,m} </math> is chosen such that the minimum value of <math> \Phi_{12}(r) </math> being <math> \Phi_{min} = - \epsilon </math>. | |||
Such forms are usually referred to as '''n-m Lennard-Jones Potential'''. | |||
For example, the [[9-3 Lennard-Jones potential |9-3 Lennard-Jones interaction potential]] is often used to model the interaction between | |||
a continuous solid wall and the atoms/molecules of a liquid. | |||
On the '9-3 Lennard-Jones potential' page a justification of this use is presented. Another example is the [[n-6 Lennard-Jones potential]], | |||
where <math>m</math> is fixed at 6, and <math>n</math> is free to adopt a range of integer values. | |||
The potentials form part of the larger class of potentials known as the [[Mie potential]]. | |||
<br> | |||
Examples: | |||
*[[8-6 Lennard-Jones potential]] | |||
*[[9-3 Lennard-Jones potential]] | |||
*[[9-6 Lennard-Jones potential]] | |||
*[[10-4-3 Lennard-Jones potential]] | |||
*[[200-100 Lennard-Jones potential]] | |||
*[[n-6 Lennard-Jones potential]] | |||
==Equation of state== | |||
:''Main article: [[Lennard-Jones equation of state]]'' | |||
== | ==Virial coefficients== | ||
:''Main article: [[Lennard-Jones model: virial coefficients]]'' | |||
==Phase diagram== | |||
:''Main article: [[Phase diagram of the Lennard-Jones model]]'' | |||
==Related models== | |||
*[[Kihara potential]] | |||
*[[Lennard-Jones model in 1-dimension]] (rods) | |||
*[[Lennard-Jones disks | Lennard-Jones model in 2-dimensions]] (disks) | |||
*[[Lennard-Jones model in 4-dimensions]] | |||
*[[Lennard-Jones sticks]] | |||
*[[Mie potential]] | |||
*[[Soft-core Lennard-Jones model]] | |||
*[[Soft sphere potential]] | |||
*[[Stockmayer potential]] | |||
;Mixtures | |||
*[[Binary Lennard-Jones mixtures]] | |||
*[[Multicomponent Lennard-Jones mixtures]] | |||
==References== | |||
<references /> | |||
[[Category:Models]] | [[Category:Models]] | ||
Latest revision as of 23:24, 30 July 2021
The Lennard-Jones intermolecular pair potential is a special case of the Mie potential and takes its name from Sir John Edward Lennard-Jones [1] [2]. The Lennard-Jones model consists of two 'parts'; a steep repulsive term, and smoother attractive term, representing the London dispersion forces [3]. The Lennard-Jones potential is probably the most extensively studied model potential today [4]. Apart from being an important model in itself, the Lennard-Jones potential frequently forms one of 'building blocks' of many force fields and molecular-based equation of state models [5]. It is worth mentioning that the 12-6 Lennard-Jones model is not the most faithful representation of the potential energy surface, but rather its use is widespread due to its computational expediency. For example, the repulsive term is maybe better described with the exp-6 potential. One of the first computer simulations using the Lennard-Jones model was undertaken by Wood and Parker in 1957 [6] in a study of liquid argon. Thermophysical property data sampled by molecular simulations since then until 2019 has been compiled, digitalized and assessed by Stephan et al [4]. This database contains about 35,000 datapoints.
Functional form[edit]
The Lennard-Jones potential is given by
or is sometimes expressed as
where
- is the intermolecular pair potential between two particles or sites
- is the value of at which
- is the well depth (energy)
- , Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle B= 4\epsilon \sigma^{6}}
- Minimum value of Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r) } at Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle r = r_{min} } ;
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \frac{r_{min}}{\sigma} = 2^{1/6} \simeq 1.12246 ... }
In reduced units:
- Density:
where Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho := N/V } (number of particles Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle N } divided by the volume Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V } )
- Temperature: Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T^* := k_B T/\epsilon }
where is the absolute temperature and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle k_B } is the Boltzmann constant
The following is a plot of the Lennard-Jones model for the Rowley, Nicholson and Parsonage parameter set [7] (Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \epsilon/k_B = }
119.8 K and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \sigma=}
0.3405 nm). See argon for other parameter sets.
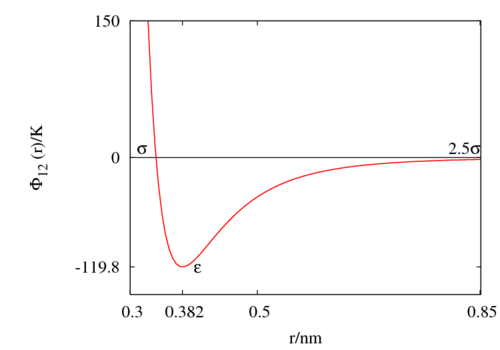
Critical point[edit]
The location of the critical point for the untruncated potential has been assessed to be at [4]
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T_c^* = 1.321 \pm 0.007 }
at a reduced density of
and the critical pressure of
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p_c^* = 0.129 \pm 0.005. }
The critical compressibility factor is given by [8]
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Z_c = \frac{p_cv_c}{RT_c} = 0.281}
Vliegenthart and Lekkerkerker [9] [10] have suggested that the critical point is related to the second virial coefficient via the expression
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle B_2 \vert_{T=T_c}= -\pi \sigma^3}
Truncated at Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 2.5 \sigma} [edit]
For the potential truncated at [11]
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T_c^* = 1.1875 (15)}
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p_c^* = 0.1105 (15)}
Triple point[edit]
The location of the triple point as found by Mastny and de Pablo [12] is
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T_{tp}^* = 0.694}
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_{tp}^* = 0.84} (liquid);
- (solid).
Radial distribution function[edit]
The following plot is of a typical radial distribution function for the monatomic Lennard-Jones liquid[13] (here with Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \sigma=3.73} Å and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \epsilon=0.294} kcal/mol at a temperature of 111.06K):
Helmholtz energy function[edit]
An expression for the Helmholtz energy function of the face centred cubic solid has been given by van der Hoef [14], applicable within the density range Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 0.94 \le \rho^* \le 1.20} and the temperature range Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 0.1 \le T^* \le 2.0} . For the liquid state see the work of Johnson, Zollweg and Gubbins [15].
Vapor-liquid equilibrium[edit]
The vapor-liquid equilibrium of the Lennard-Jones potential has been studied more than 45 times in the literature. Several of these data sets were found to have gross deviations to an entity of data sets, which is both thermodynamically consistent and in good mutual agreement [4]. The mutual agreement of these data sets was found to be approximately for the vapor pressure, Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \pm 0.75%} for the enthalpy of vaporization, Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \pm 0.2%} for the saturated liquid density, and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \pm 1%} for the saturated vapor density [4].
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \ln p^s = n_1 T + \frac{n_2}{T} + \frac{n_3}{T^{n_4}} ~~ \mathrm{with} ~~ n_i =[ 1.156551, -4.431519, -0.423028, 2.638743 ] }
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \ln \Big(\frac{\rho''}{\rho_\mathrm{c}}\Big) = \sum_{i=1}^{5} n_i\, \Big(1 - \frac{T}{T_\mathrm{c}} \Big)^{t_i} ~~ \mathrm{with}~~ n_i = [-8.135822, -102.91911, -3.037979, -44.381841, -34.55892948] ~~\& ~~ t_i = [1.651685, 43.469214, 0.462877, 11.500462, 5.39437] }
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Delta h_v = \sum_{i=1}^{4} n_i\, (T_\mathrm{c} - T)^{t_i} ~~ \mathrm{with} ~~ n_i = [6.456728, 2.700099, -3.073573, 3.149052 ] ~~\& ~~ t_i = [0.411342, 0.460416, 2.350953, 5.01701 ] }
Melting line[edit]
The solid and liquid densities along the melting line [16] are given by the following equations:
van der Hoef[edit]
van der Hoef (Ref. [17] Eqs. 25 and 26):
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_{\mathrm {solid}} = \beta^{-1/4} \left[ 0.92302 - 0.09218 \beta + 0.62381 \beta^2 -0.82672 \beta^3 + 0.49124 \beta^4 -0.10847 \beta^5\right]}
and
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_{\mathrm {liquid}} = \beta^{-1/4} \left[ 0.91070 - 0.25124 \beta + 0.85861 \beta^2 -1.08918 \beta^3 + 0.63932 \beta^4 -0.14433 \beta^5\right]}
Mastny and de Pablo[edit]
Mastny and de Pablo (Ref [18] Eqs. 20 and 21):
and
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_{\mathrm {liquid}} = \beta^{-1/4} \left[ 0.90735 - 0.27120 \beta + 0.91784 \beta^2 -1.16270\beta^3 + 0.68012 \beta^4 -0.15284 \beta^5\right]}
A study has been performed of the solid-fluid equilibria, and behavior in the high density region [19].
Zeno line[edit]
It has been shown that the Lennard-Jones model has a straight Zeno line [20] on the density-temperature plane.
Widom line[edit]
It has been shown that the Lennard-Jones model has a Widom line [21] on the pressure-temperature plane.
Viscosity[edit]
Perturbation theory[edit]
The Lennard-Jones model is also used in perturbation theories, for example see: Weeks-Chandler-Andersen perturbation theory.
Approximations in simulation: truncation and shifting[edit]
The Lennard-Jones model is often used with a cutoff radius of Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 2.5 \sigma} , beyond which Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r)} is set to zero. Setting the well depth Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \epsilon } to be 1 in the potential on arrives at , i.e. at this distance the potential is at less than 2% of the well depth. For an analysis of the effect of this cutoff on the melting line see the work of Mastny and de Pablo [12] and of Ahmed and Sadus [23]. See Panagiotopoulos for critical parameters [24]. See Pártay for the ground state structure [25]. It has recently been suggested that a truncated and shifted force cutoff of Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 1.5 \sigma} can be used under certain conditions [26]. In order to avoid any discontinuity, a piecewise continuous version, known as the modified Lennard-Jones model, was developed.
Cutoff Lennard-Jones potential[edit]
The cutoff Lennard-Jones potential is given by (Eq. 2 in [27]):
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r) = 4 \epsilon \left\{ \left[ \left(\frac{\sigma}{r} \right)^{12}- \left( \frac{\sigma}{r}\right)^6 \right]+ \left[ 6\left(\frac{\sigma}{r_c} \right)^{12}- 3\left( \frac{\sigma}{r_c}\right)^6 \right] \left(\frac{r}{r_c} \right)^2 -7 \left(\frac{\sigma}{r_c} \right)^{12} + 4 \left(\frac{\sigma}{r_c} \right)^{6} \right\}}
where Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle r_c} is the cutoff radius.
n-m Lennard-Jones potential[edit]
It is relatively common to encounter potential functions given by:
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r) = c_{n,m} \epsilon \left[ \left( \frac{ \sigma }{r } \right)^n - \left( \frac{\sigma}{r} \right)^m \right]. }
with and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m }
being positive integers and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle n > m }
.
Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle c_{n,m} }
is chosen such that the minimum value of Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r) }
being .
Such forms are usually referred to as n-m Lennard-Jones Potential.
For example, the 9-3 Lennard-Jones interaction potential is often used to model the interaction between
a continuous solid wall and the atoms/molecules of a liquid.
On the '9-3 Lennard-Jones potential' page a justification of this use is presented. Another example is the n-6 Lennard-Jones potential,
where Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m}
is fixed at 6, and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle n}
is free to adopt a range of integer values.
The potentials form part of the larger class of potentials known as the Mie potential.
Examples:
- 8-6 Lennard-Jones potential
- 9-3 Lennard-Jones potential
- 9-6 Lennard-Jones potential
- 10-4-3 Lennard-Jones potential
- 200-100 Lennard-Jones potential
- n-6 Lennard-Jones potential
Equation of state[edit]
- Main article: Lennard-Jones equation of state
Virial coefficients[edit]
- Main article: Lennard-Jones model: virial coefficients
Phase diagram[edit]
- Main article: Phase diagram of the Lennard-Jones model
Related models[edit]
- Kihara potential
- Lennard-Jones model in 1-dimension (rods)
- Lennard-Jones model in 2-dimensions (disks)
- Lennard-Jones model in 4-dimensions
- Lennard-Jones sticks
- Mie potential
- Soft-core Lennard-Jones model
- Soft sphere potential
- Stockmayer potential
- Mixtures
References[edit]
- ↑ John Edward Lennard-Jones "On the Determination of Molecular Fields. I. From the Variation of the Viscosity of a Gas with Temperature", Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 106 pp. 441-462 (1924) § 8 (ii)
- ↑ John Edward Lennard-Jones "On the Determination of Molecular Fields. II. From the Equation of State of a Gas", Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 106 pp. 463-477 (1924) Eq. 2.05
- ↑ F. London "Zur Theorie und Systematik der Molekularkräfte", Zeitschrift für Physik A Hadrons and Nuclei 63 pp. 245-279 (1930)
- ↑ 4.0 4.1 4.2 4.3 4.4 Simon Stephan, Monika Thol, Jadran Vrabec, and Hans Hasse "Thermophysical Properties of the Lennard-Jones Fluid: Database and Data Assessment", Journal of Chemical Information and Modeling 59, 10 pp. 4248–4265 (2019)
- ↑ Simon Stephan, Jens Staubach, Hans Hasse "Review and comparison of equations of state for the Lennard-Jones fluid", Fluid Phase Equilibria 523 pp. 112772 (2020)
- ↑ W. W. Wood and F. R. Parker "Monte Carlo Equation of State of Molecules Interacting with the Lennard‐Jones Potential. I. A Supercritical Isotherm at about Twice the Critical Temperature", Journal of Chemical Physics 27 pp. 720- (1957)
- ↑ L. A. Rowley, D. Nicholson and N. G. Parsonage "Monte Carlo grand canonical ensemble calculation in a gas-liquid transition region for 12-6 Argon", Journal of Computational Physics 17 pp. 401-414 (1975)
- ↑ V. L. Kulinskii "The critical compressibility factor of fluids from the global isomorphism approach", Journal of Chemical Physics 139 184119 (2013)
- ↑ G. A. Vliegenthart and H. N. W. Lekkerkerker "Predicting the gas–liquid critical point from the second virial coefficient", Journal of Chemical Physics 112 pp. 5364-5369 (2000)
- ↑ L. A. Bulavin and V. L. Kulinskii "Generalized principle of corresponding states and the scale invariant mean-field approach", Journal of Chemical Physics '133 134101 (2010)
- ↑ Ernesto S. Loscar, C. Gastón Ferrara1, and Tomás S. Grigera "Spinodals and critical point using short-time dynamics for a simple model of liquid", Journal of Chemical Physics 144 134501 (2016)
- ↑ 12.0 12.1 Ethan A. Mastny and Juan J. de Pablo "Melting line of the Lennard-Jones system, infinite size, and full potential", Journal of Chemical Physics 127 104504 (2007)
- ↑ John G. Kirkwood, Victor A. Lewinson, and Berni J. Alder "Radial Distribution Functions and the Equation of State of Fluids Composed of Molecules Interacting According to the Lennard-Jones Potential", Journal of Chemical Physics 20 pp. 929- (1952)
- ↑ Martin A. van der Hoef "Free energy of the Lennard-Jones solid", Journal of Chemical Physics 113 pp. 8142-8148 (2000)
- ↑ J. Karl Johnson, John A. Zollweg and Keith E. Gubbins "The Lennard-Jones equation of state revisited", Molecular Physics 78 pp. 591-618 (1993)
- ↑ D. M. Heyes and A. C. Brańka "The Lennard-Jones melting line and isomorphism", Journal of Chemical Physics 143 234504 (2015)
- ↑ Martin A. van der Hoef "Free energy of the Lennard-Jones solid", Journal of Chemical Physics 113 pp. 8142-8148 (2000)
- ↑ Ethan A. Mastny and Juan J. de Pablo "Melting line of the Lennard-Jones system, infinite size, and full potential", Journal of Chemical Physics 127 104504 (2007)
- ↑ Andreas Köster, Peter Mausbach, and Jadran Vrabec "Premelting, solid-fluid equilibria, and thermodynamic properties in the high density region based on the Lennard-Jones potential", Journal of Chemical Physics 147 144502 (2017)
- ↑ E. M. Apfelbaum, V. S. Vorob’ev and G. A. Martynov "Regarding the Theory of the Zeno Line", Journal of Physical Chemistry A 112 pp. 6042-6044 (2008)
- ↑ V. V. Brazhkin, Yu. D. Fomin, A. G. Lyapin, V. N. Ryzhov, and E. N. Tsiok "Widom Line for the Liquid–Gas Transition in Lennard-Jones System", Journal of Physical Chemistry B Article ASAP (2011)
- ↑ Kai-Min Tu,  Kang Kim, and  Nobuyuki Matubayasi "Spatial-decomposition analysis of viscosity with application to Lennard-Jones fluid", Journal of Chemical Physics 148 094501 (2018)
- ↑ Alauddin Ahmed and Richard J. Sadus "Effect of potential truncations and shifts on the solid-liquid phase coexistence of Lennard-Jones fluids", Journal of Chemical Physics 133 124515 (2010)
- ↑ A. Z. Panagiotopoulos "Molecular simulation of phase coexistence: Finite-size effects and determination of critical parameters for two- and three-dimensional Lennard-Jones fluids", International Journal of Thermophysics 15 pp. 1057-1072 (1994)
- ↑ Lívia B. Pártay, Christoph Ortner, Albert P. Bartók, Chris J. Pickard and Gábor Csányi "Polytypism in the ground state structure of the Lennard-Jonesium", Physical Chemistry Chemical Physics 19 19369 (2017)
- ↑ Søren Toxvaerd and Jeppe C. Dyre "Communication: Shifted forces in molecular dynamics", Journal of Chemical Physics 134 081102 (2011)
- ↑ Spotswood D. Stoddard and Joseph Ford "Numerical Experiments on the Stochastic Behavior of a Lennard-Jones Gas System", Physical Review A 8 pp. 1504-1512 (1973)
![{\displaystyle \Phi _{12}(r)=4\epsilon \left[\left({\frac {\sigma }{r}}\right)^{12}-\left({\frac {\sigma }{r}}\right)^{6}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bc9f91d43a2f39f321b2054db022e07ae7c29988)














![{\displaystyle {\Big (}{\frac {\rho '}{\rho _{\mathrm {c} }}}{\Big )}=1+\sum _{i=1}^{5}n_{i}\,{\Big (}1-{\frac {T}{T_{\mathrm {c} }}}{\Big )}^{t_{i}}~~\mathrm {with} ~~n_{i}=[1.3417,2.075332,-2.123475,0.328998,1.386131]~~\&~~t_{i}=[0.32714,0.958759,1.645654,17.000001,2.400858]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/579bba58f1e070993261409af3923aacdb7fbf37)
![{\displaystyle \rho _{\mathrm {solid} }=\beta ^{-1/4}\left[0.908629-0.041510\beta +0.514632\beta ^{2}-0.708590\beta ^{3}+0.428351\beta ^{4}-0.095229\beta ^{5}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9d42739dd208f4ac0bb02525ea454ca8598593fd)


