TIP4P/2005 model of water: Difference between revisions
Carl McBride (talk | contribs) m (→Phase diagram) |
Carl McBride (talk | contribs) m (→Supercooled region: Added a recent publication) |
||
| Line 31: | Line 31: | ||
Recent simulations have suggested the possibility of a [[Plastic crystals | plastic crystal]] phase or phases for water <ref name="multiple2"> </ref><ref>[http://dx.doi.org/10.1039/b812834k J. L. Aragones, M. M. Conde, E. G. Noya and C. Vega "The phase diagram of water at high pressures as obtained by computer simulations of the TIP4P/2005 model: the appearance of a plastic crystal phase", Physical Chemistry Chemical Physics '''11''' pp. 543- (2009)]</ref> | Recent simulations have suggested the possibility of a [[Plastic crystals | plastic crystal]] phase or phases for water <ref name="multiple2"> </ref><ref>[http://dx.doi.org/10.1039/b812834k J. L. Aragones, M. M. Conde, E. G. Noya and C. Vega "The phase diagram of water at high pressures as obtained by computer simulations of the TIP4P/2005 model: the appearance of a plastic crystal phase", Physical Chemistry Chemical Physics '''11''' pp. 543- (2009)]</ref> | ||
====Supercooled region==== | ====Supercooled region==== | ||
The [[Supercooling and nucleation | supercooled]] region has been studied by Abascal and Vega <ref>[http://dx.doi.org/10.1063/1.3585676 J. L. F. Abascal and C. Vega "Note: Equation of state and compressibility of supercooled water: Simulations and experiment", Journal of Chemical Physics '''134''' 186101 (2011)]</ref>, locating a [[Widom line]], indicating a [[second critical point for water]] located at 1350 bar and 193 K. | The [[Supercooling and nucleation | supercooled]] region has been studied by Abascal and Vega <ref>[http://dx.doi.org/10.1063/1.3585676 J. L. F. Abascal and C. Vega "Note: Equation of state and compressibility of supercooled water: Simulations and experiment", Journal of Chemical Physics '''134''' 186101 (2011)]</ref>, locating a [[Widom line]] <ref>[http://dx.doi.org/10.1063/1.3594545 K. T. Wikfeldt, C. Huang, A. Nilsson, and L. G. M. Pettersson "Enhanced small-angle scattering connected to the Widom line in simulations of supercooled water", Journal of Chemical Physics '''134''' 214506 (2011)]</ref>, indicating a [[second critical point for water]] located at 1350 bar and 193 K. | ||
==Surface tension== | ==Surface tension== | ||
Revision as of 12:10, 7 June 2011
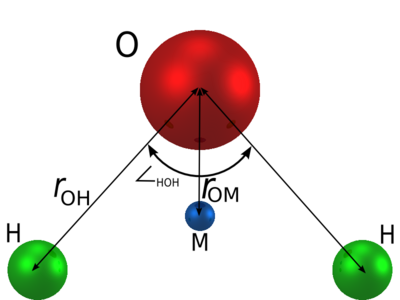
The TIP4P/2005 model [1] is a re-parameterisation of the original TIP4P potential for simulations of water. TIP4P/2005 is a rigid planar model, having a similar geometry to that of the Bernal and Fowler model.
Parameters
The TIP4P/2005 model consists of a Lennard-Jones site for the oxygen atom, and three charge sites.
| (Å) | HOH , deg | (Å) | (K) | q(O) (e) | q(H) (e) | q(M) (e) | (Å) |
| 0.9572 | 104.52 | 3.1589 | 93.2 | 0 | 0.5564 | -2q(H) | 0.1546 |
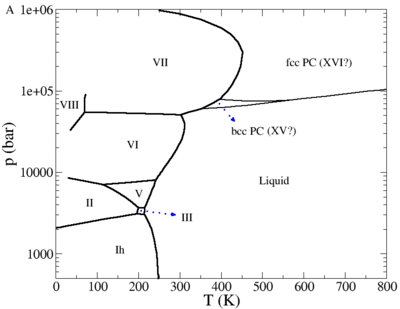
Phase diagram
The phase diagram of the TIP4P/2005 model in the pressure-temperature plane (adapted from Fig. 9a of [2]) is given in a publication by Abascal, Sanz and Vega [3]
and for negative pressures in the publication [4]
Liquid-vapour equilibria
Plastic crystal phases
Recent simulations have suggested the possibility of a plastic crystal phase or phases for water [2][6]
Supercooled region
The supercooled region has been studied by Abascal and Vega [7], locating a Widom line [8], indicating a second critical point for water located at 1350 bar and 193 K.
Surface tension
The surface tension has been studied for the TIP4P/2005 model, giving mJ/m2 at 300 K using the test area method [9], mN/m at 300 K using using the Ewald sums for the dispersion interactions [10], mJ/m2 at 350 K using the reaction field method [11] and mN/m at 249 K [12].
Self-diffusion coefficient
The TIP4P/2005 potential has a self-diffusion coefficient, in bulk water at 298 K, of 0.21 Å2 ps−1 in a classical simulation of 216 water molecules (experimental value: 0.23 Å2 ps−1) [13].
Shear viscosity
The shear viscosity for the TIP4P/2005 model is 0.855 mPa.s at 298 K and 1 bar [14] (experimental value 0.896 mPa.s [15]).
Liquid-liquid critical point
For the TIP4P/2005 model the liquid-liquid critical point is located at K, bar and having a density of g/cm3 [16].
Structure factor
The structure factor, in particular for small wave vectors, has been calculated by Sedlmeier, Horinek and Netz [17], who observe that the TIP4P/2005 model yields an "almost quantitative agreement".
Virial coefficients
The second virial coefficient has been calculated by Chialvo et al [18].
References
- ↑ J. L. F. Abascal and C. Vega "A general purpose model for the condensed phases of water: TIP4P/2005", Journal of Chemical Physics, 123 234505 (2005)
- ↑ 2.0 2.1 J. L. Aragones and C. Vega "Plastic crystal phases of simple water models", Journal of Chemical Physics 130 244504 (2009)
- ↑ Jose L. F. Abascal, Eduardo Sanz and Carlos Vega "Triple points and coexistence properties of the dense phases of water calculated using computer simulation", Physical Chemistry Chemical Physics 11 pp. 556-562 (2009)
- ↑ M. M. Conde, C. Vega, G. A. Tribello, and B. Slater "The phase diagram of water at negative pressures: Virtual ices", Journal of Chemical Physics 131 034510 (2009)
- ↑ C. Vega, J. L. F. Abascal and I. Nezbeda "Vapor-liquid equilibria from the triple point up to the critical point for the new generation of TIP4P-like models: TIP4P/Ew, TIP4P/2005, and TIP4P/ice" Journal of Chemical Physics 125 034503 (2006)
- ↑ J. L. Aragones, M. M. Conde, E. G. Noya and C. Vega "The phase diagram of water at high pressures as obtained by computer simulations of the TIP4P/2005 model: the appearance of a plastic crystal phase", Physical Chemistry Chemical Physics 11 pp. 543- (2009)
- ↑ J. L. F. Abascal and C. Vega "Note: Equation of state and compressibility of supercooled water: Simulations and experiment", Journal of Chemical Physics 134 186101 (2011)
- ↑ K. T. Wikfeldt, C. Huang, A. Nilsson, and L. G. M. Pettersson "Enhanced small-angle scattering connected to the Widom line in simulations of supercooled water", Journal of Chemical Physics 134 214506 (2011)
- ↑ C. Vega and E. de Miguel "Surface tension of the most popular models of water by using the test-area simulation method", Journal of Chemical Physics 126 154707 (2007)
- ↑ José Alejandre and Gustavo A. Chapela "The surface tension of TIP4P/2005 water model using Ewald sums for the dispersion interactions", Journal of Chemical Physics 132 014701 (2010)
- ↑ J. M. Míguez, D. González-Salgado, J. L. Legido, and M. M. Piñeiro "Calculation of interfacial properties using molecular simulation with the reaction field method: Results for different water models", Journal of Chemical Physics 132 184102 (2010)
- ↑ Ryuji Sakamaki, Amadeu K. Sum, Tetsu Narumi, and Kenji Yasuoka "Molecular dynamics simulations of vapor/liquid coexistence using the nonpolarizable water models", Journal of Chemical Physics 134 124708 (2011)
- ↑ Thomas E. Markland, Scott Habershon, and David E. Manolopoulos "Quantum diffusion of hydrogen and muonium atoms in liquid water and hexagonal ice", Journal of Chemical Physics 128 194506 (2008)
- ↑ Miguel Angel González and José L. F. Abascal "The shear viscosity of rigid water models", Journal of Chemical Physics 132 096101 (2010)
- ↑ Kenneth R. Harris and Lawrence A. Woolf "Temperature and Volume Dependence of the Viscosity of Water and Heavy Water at Low Temperatures", Journal of Chemical & Engineering Data 49 pp. 1064-1069 (2004)
- ↑ José L. F. Abascal and Carlos Vega "Widom line and the liquid–liquid critical point for the TIP4P/2005 water model", Journal of Chemical Physics 133 234502 (2010)
- ↑ Felix Sedlmeier, Dominik Horinek, and Roland R. Netz "Spatial Correlations of Density and Structural Fluctuations in Liquid Water: A Comparative Simulation Study", Journal of the American Chemical Society Article ASAP (2011)
- ↑ Ariel A. Chialvo, Albert Bartók and András Baranyai "On the re-engineered TIP4P water models for the prediction of vapor–liquid equilibrium", Journal of Molecular Liquids 129 pp. 120-124 (2006)
Related reading