Lennard-Jones model: Difference between revisions
Carl McBride (talk | contribs) m (→Related models: Added an internal link to the n-6 Lennard-Jones potential) |
Carl McBride (talk | contribs) m (→m-n Lennard-Jones potential: Interchanged n for m to keep in line with other entries and publications.) |
||
| Line 50: | Line 50: | ||
for an analysis of the effect of this cutoff on the melting line. | for an analysis of the effect of this cutoff on the melting line. | ||
== m | == n-m Lennard-Jones potential == | ||
It is relatively common to encounter potential functions given by: | It is relatively common to encounter potential functions given by: | ||
: <math> \Phi_{12}(r) = c_{m | : <math> \Phi_{12}(r) = c_{n,m} \epsilon \left[ \left( \frac{ \sigma }{r } \right)^n - \left( \frac{\sigma}{r} \right)^m | ||
\right]. | \right]. | ||
</math> | </math> | ||
with <math> | with <math> n </math> and <math> m </math> being positive integers and <math> n > m </math>. | ||
<math> c_{m | <math> c_{n,m} </math> is chosen such that the minimum value of <math> \Phi_{12}(r) </math> being <math> \Phi_{min} = - \epsilon </math>. | ||
Such forms are usually referred to as '''m | Such forms are usually referred to as '''n-m Lennard-Jones Potential'''. | ||
For example, the [[9-3 Lennard-Jones potential |9-3 Lennard-Jones interaction potential]] is often used to model the interaction between | For example, the [[9-3 Lennard-Jones potential |9-3 Lennard-Jones interaction potential]] is often used to model the interaction between | ||
the atoms/molecules of a fluid and a continuous solid wall. | the atoms/molecules of a fluid and a continuous solid wall. | ||
On the '9-3 Lennard-Jones potential' page a justification of this use is presented. | On the '9-3 Lennard-Jones potential' page a justification of this use is presented. Another example is the [[n-6 Lennard-Jones potential]], | ||
where <math>m</math> is fixed at 6, and <math>n</math> is free to adopt a range of integer values. | |||
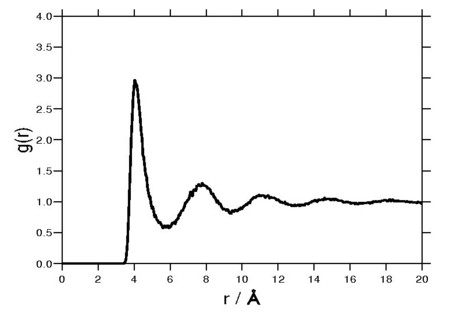
==Radial distribution function== | ==Radial distribution function== | ||
The following plot is of a typical [[radial distribution function]] for the monatomic Lennard-Jones liquid (here with <math>\sigma=3.73 {\mathrm {\AA}}</math> and <math>\epsilon=0.294</math> kcal/mol at a [[temperature]] of 111.06K): | The following plot is of a typical [[radial distribution function]] for the monatomic Lennard-Jones liquid (here with <math>\sigma=3.73 {\mathrm {\AA}}</math> and <math>\epsilon=0.294</math> kcal/mol at a [[temperature]] of 111.06K): | ||
Revision as of 16:19, 3 November 2009
The Lennard-Jones intermolecular pair potential is a special case of the Mie potential and takes its name from Sir John Edward Lennard-Jones [1]. The Lennard-Jones model consists of two 'parts'; a steep repulsive term, and smoother attractive term, representing the London dispersion forces. Apart from being an important model in its-self, the Lennard-Jones potential frequently forms one of 'building blocks' of may force fields,
Functional form
The Lennard-Jones potential is given by
where
- is the intermolecular pair potential between two particles or sites
- is the diameter (length), i.e. the value of at which
- is the well depth (energy)
In reduced units:
- Density: , where (number of particles divided by the volume )
- Temperature: , where is the absolute temperature and is the Boltzmann constant
The following is a plot of the Lennard-Jones model for the parameters 120 K and 0.34 nm. See argon for different parameter sets.
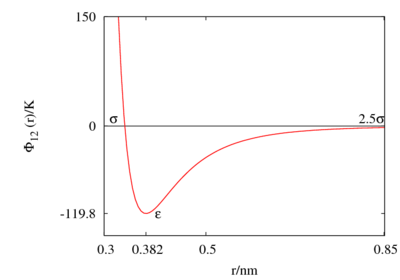
This figure was produced using gnuplot with the command:
plot (4*120*((0.34/x)**12-(0.34/x)**6))
Special points
- Minimum value of at Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle r = r_{min} } ;
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \frac{r_{min}}{\sigma} = 2^{1/6} \simeq 1.12246 ... }
Critical point
The location of the critical point is [2]
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T_c^* = 1.326 \pm 0.002}
at a reduced density of
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_c^* = 0.316 \pm 0.002} .
Vliegenthart and Lekkerkerker [3] have suggested that the critical point is related to the second virial coefficient via the expression
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle B_2 \vert_{T=T_c}= -\pi \sigma^3}
Triple point
The location of the triple point as found by Mastny and de Pablo (Ref. 4) is
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T_{tp}^* = 0.694}
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_{tp}^* = 0.84} (liquid); Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_{tp}^* = 0.96} (solid)
Approximations in simulation: truncation and shifting
The Lennard-Jones model is often used with a cutoff radius of Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 2.5 \sigma} , beyond which Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r)} is set to zero. See Mastny and de Pablo [4] for an analysis of the effect of this cutoff on the melting line.
n-m Lennard-Jones potential
It is relatively common to encounter potential functions given by:
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r) = c_{n,m} \epsilon \left[ \left( \frac{ \sigma }{r } \right)^n - \left( \frac{\sigma}{r} \right)^m \right]. }
with and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m } being positive integers and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle n > m } . Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle c_{n,m} } is chosen such that the minimum value of Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{12}(r) } being Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Phi_{min} = - \epsilon } . Such forms are usually referred to as n-m Lennard-Jones Potential. For example, the 9-3 Lennard-Jones interaction potential is often used to model the interaction between the atoms/molecules of a fluid and a continuous solid wall. On the '9-3 Lennard-Jones potential' page a justification of this use is presented. Another example is the n-6 Lennard-Jones potential, where Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle m} is fixed at 6, and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle n} is free to adopt a range of integer values.
Radial distribution function
The following plot is of a typical radial distribution function for the monatomic Lennard-Jones liquid (here with Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \sigma=3.73 {\mathrm {\AA}}} and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \epsilon=0.294} kcal/mol at a temperature of 111.06K):
Equation of state
- Main article: Lennard-Jones equation of state
Virial coefficients
- Main article: Lennard-Jones model: virial coefficients
Phase diagram
- Main article: Phase diagram of the Lennard-Jones model
Perturbation theory
The Lennard-Jones model is also used in perturbation theories, for example see: Weeks-Chandler-Anderson perturbation theory.
Mixtures
Related models
- 9-3 Lennard-Jones potential
- 10-4-3 Lennard-Jones potential
- n-6 Lennard-Jones potential
- Lennard-Jones model in 1-dimension (rods)
- Lennard-Jones model in 2-dimensions (disks)
- Lennard-Jones model in 4-dimensions
- Lennard-Jones sticks
- Mie potential
- Soft sphere potential
- Stockmayer potential
References
- ↑ J. E. Lennard-Jones, "Cohesion", Proceedings of the Physical Society, 43 pp. 461-482 (1931)
- ↑ J. M. Caillol " Critical-point of the Lennard-Jones fluid: A finite-size scaling study", Journal of Chemical Physics 109 pp. 4885-4893 (1998)
- ↑ G. A. Vliegenthart and H. N. W. Lekkerkerker "Predicting the gas–liquid critical point from the second virial coefficient", Journal of Chemical Physics 112 pp. 5364-5369 (2000)
- ↑ Ethan A. Mastny and Juan J. de Pablo "Melting line of the Lennard-Jones system, infinite size, and full potential", Journal of Chemical Physics 127 104504 (2007)
![{\displaystyle \Phi _{12}(r)=4\epsilon \left[\left({\frac {\sigma }{r}}\right)^{12}-\left({\frac {\sigma }{r}}\right)^{6}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bc9f91d43a2f39f321b2054db022e07ae7c29988)
















