Lennard-Jones model: Difference between revisions
Carl McBride (talk | contribs) m (→Functional form: Added internal link) |
Carl McBride (talk | contribs) |
||
| Line 9: | Line 9: | ||
* <math> \Phi(r) </math> is the [[intermolecular pair potential]] between two particles at a distance r; | * <math> \Phi(r) </math> is the [[intermolecular pair potential]] between two particles at a distance r; | ||
* <math> \sigma </math> | * <math> \sigma </math> is the diameter (length), i.e. the value of <math>r</math> at <math> \Phi(r)=0</math> ; | ||
* <math> \epsilon </math> : well depth (energy) | * <math> \epsilon </math> : well depth (energy) | ||
Revision as of 12:51, 4 January 2008
The Lennard-Jones intermolecular pair potential was developed by Sir John Edward Lennard-Jones in 1931 (Ref. 1).
Functional form
The Lennard-Jones potential is given by:
where:
- is the intermolecular pair potential between two particles at a distance r;
- is the diameter (length), i.e. the value of at ;
- : well depth (energy)
Reduced units:
- Density, , where (number of particles divided by the volume .)
- Temperature; , where is the absolute temperature and is the Boltzmann constant
Argon
The Lennard-Jones parameters for argon are 119.8 K and 0.3405 nm. (Ref. ?)
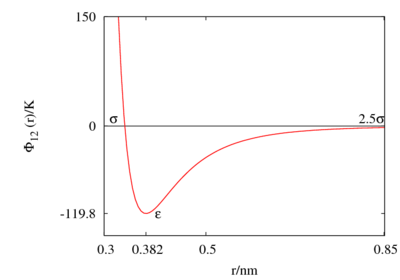
This figure was produced using gnuplot with the command:
plot (4*120*((0.34/x)**12-(0.34/x)**6))
Features
Special points:
- Minimum value of at ;
Critical point
The location of the critical point is (Caillol (Ref. 3))
at a reduced density of
- .
Triple point
The location of the triple point as found by Mastny and de Pablo (Ref. 2) is
Approximations in simulation: truncation and shifting
The Lennard-Jones model is often used with a cutoff radius of . See Mastny and de Pablo (Ref. 2) for an analysis of the effect of this cutoff on the melting line.
m-n Lennard-Jones potential
It is relatively common to encounter potential functions given by:
with and being positive integers and . is chosen such that the minimum value of being . Such forms are usually referred to as m-n Lennard-Jones Potential. For example, the 9-3 Lennard-Jones interaction potential is often used to model the interaction between the atoms/molecules of a fluid and a continuous solid wall. On the '9-3 Lennard-Jones potential' page a justification of this use is presented.
Related pages
- Phase diagram of the Lennard-Jones model
- Lennard-Jones model: virial coefficients
- Lennard-Jones equation of state
- Lennard-Jones sticks
- Lennard-Jones disks
- 9-3 Lennard-Jones potential
References
- J. E. Lennard-Jones, "Cohesion", Proceedings of the Physical Society, 43 pp. 461-482 (1931)
- Ethan A. Mastny and Juan J. de Pablo "Melting line of the Lennard-Jones system, infinite size, and full potential", Journal of Chemical Physics 127 104504 (2007)
- J. M. Caillol " Critical-point of the Lennard-Jones fluid: A finite-size scaling study", Journal of Chemical Physics 109 pp. 4885-4893 (1998)
![{\displaystyle \Phi (r)=4\epsilon \left[\left({\frac {\sigma }{r}}\right)^{12}-\left({\frac {\sigma }{r}}\right)^{6}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b0ecd4ec335b5149b5f30ba2d02a6049d0407207)





















![{\displaystyle \Phi (r)=c_{m,n}\epsilon \left[\left({\frac {\sigma }{r}}\right)^{m}-\left({\frac {\sigma }{r}}\right)^{n}\right].}](https://wikimedia.org/api/rest_v1/media/math/render/svg/58317a77137d2a636f30e2a4a48c2ba87b516598)




