Lennard-Jones model: Difference between revisions
m (→Critical point) |
Carl McBride (talk | contribs) m (Slight tidy up.) |
||
| Line 1: | Line 1: | ||
The '''Lennard-Jones''' potential was developed by [[ Sir John Edward Lennard-Jones KBE, FRS | Sir John Edward Lennard-Jones]]. | The '''Lennard-Jones''' [[intermolecular pair potential]] was developed by [[ Sir John Edward Lennard-Jones KBE, FRS | Sir John Edward Lennard-Jones]]. | ||
== Lennard-Jones potential == | == Lennard-Jones potential == | ||
The Lennard-Jones potential is given by: | The Lennard-Jones potential is given by: | ||
| Line 21: | Line 21: | ||
==Argon== | ==Argon== | ||
The Lennard-Jones parameters for argon are <math>\epsilon/k_B \approx</math> 119.8 K and <math>\sigma \approx</math> 0.3405 nm. (Ref. ?) | The Lennard-Jones parameters for argon are <math>\epsilon/k_B \approx</math> 119.8 K and <math>\sigma \approx</math> 0.3405 nm. (Ref. ?) | ||
[[Image:Lennard-Jones.png|center]] | [[Image:Lennard-Jones.png|400px|center]] | ||
This figure was produced using [http://www.gnuplot.info/ gnuplot] with the command: | This figure was produced using [http://www.gnuplot.info/ gnuplot] with the command: | ||
| Line 27: | Line 27: | ||
== Features == | == Features == | ||
Special points: | Special points: | ||
* <math> \Phi(\sigma) = 0 </math> | * <math> \Phi(\sigma) = 0 </math> | ||
* Minimum value of <math> \Phi(r) </math> at <math> r = r_{min} </math>; | * Minimum value of <math> \Phi(r) </math> at <math> r = r_{min} </math>; | ||
: <math> \frac{r_{min}}{\sigma} = 2^{1/6} \simeq 1.12246 ... </math> | : <math> \frac{r_{min}}{\sigma} = 2^{1/6} \simeq 1.12246 ... </math> | ||
====Critical point==== | ====Critical point==== | ||
The location of the [[Critical points |critical point]] is (Caillol (Ref. 3)) | The location of the [[Critical points |critical point]] is (Caillol (Ref. 3)) | ||
| Line 41: | Line 36: | ||
at a reduced density of | at a reduced density of | ||
:<math>\rho_c^* = 0.316 \pm 0.002</math>. | :<math>\rho_c^* = 0.316 \pm 0.002</math>. | ||
====Triple point==== | ====Triple point==== | ||
The location of the [[triple point]] as found by Mastny and de Pablo (Ref. 2) is | The location of the [[triple point]] as found by Mastny and de Pablo (Ref. 2) is | ||
| Line 50: | Line 44: | ||
fa an analysis of the effect of this cutoff on the melting line. | fa an analysis of the effect of this cutoff on the melting line. | ||
== Related potential models == | == Related potential models == | ||
It is relatively common the use of potential functions given by: | It is relatively common the use of potential functions given by: | ||
: <math> \Phi (r) = c_{m,n} \epsilon \left[ \left( \frac{ \sigma }{r } \right)^m - \left( \frac{\sigma}{r} \right)^n | : <math> \Phi (r) = c_{m,n} \epsilon \left[ \left( \frac{ \sigma }{r } \right)^m - \left( \frac{\sigma}{r} \right)^n | ||
\right]. | \right]. | ||
</math> | </math> | ||
with <math> m </math> and <math> n </math> being positive integer numbers and <math> m > n </math>, and | with <math> m </math> and <math> n </math> being positive integer numbers and <math> m > n </math>, and | ||
<math> c_{m,n} </math> is chosen to get the minimum value of <math> \Phi(r) </math> being <math> \Phi_{min} = - \epsilon </math> | <math> c_{m,n} </math> is chosen to get the minimum value of <math> \Phi(r) </math> being <math> \Phi_{min} = - \epsilon </math>. | ||
Such forms are usually referred to as '''m-n Lennard-Jones Potential'''. | |||
The [[9-3 Lennard-Jones potential |9-3 Lennard-Jones interaction potential]] is often use to model the interaction between | |||
The 9-3 Lennard-Jones interaction potential is often use to model the interaction between | |||
the atoms/molecules of a fluid and a continuous solid wall. | the atoms/molecules of a fluid and a continuous solid wall. | ||
On the '9-3 Lennard-Jones potential' page a justification of this use is presented. | |||
====Other dimensions==== | ====Other dimensions==== | ||
* 1-dimensional case: [[Lennard-Jones rods]]. | * 1-dimensional case: [[Lennard-Jones rods]]. | ||
| Line 72: | Line 61: | ||
*[[Lennard-Jones model: virial coefficients]] | *[[Lennard-Jones model: virial coefficients]] | ||
*[[Lennard-Jones equation of state]] | *[[Lennard-Jones equation of state]] | ||
*[[Lennard-Jones sticks]] | |||
==References== | ==References== | ||
Revision as of 17:14, 23 November 2007
The Lennard-Jones intermolecular pair potential was developed by Sir John Edward Lennard-Jones.
Lennard-Jones potential
The Lennard-Jones potential is given by:
where:
- is the intermolecular pair potential between two particles at a distance r;
- : diameter (length);
- : well depth (energy)
Reduced units:
- Density, , where (number of particles divided by the volume .)
- Temperature; , where is the absolute temperature and is the Boltzmann constant
Argon
The Lennard-Jones parameters for argon are 119.8 K and 0.3405 nm. (Ref. ?)
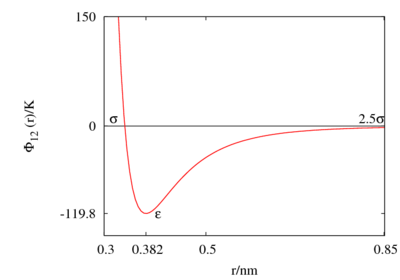
This figure was produced using gnuplot with the command:
plot (4*120*((0.34/x)**12-(0.34/x)**6))
Features
Special points:
- Minimum value of at ;
Critical point
The location of the critical point is (Caillol (Ref. 3))
at a reduced density of
- .
Triple point
The location of the triple point as found by Mastny and de Pablo (Ref. 2) is
Approximations in simulation: truncation and shifting
The Lennard-Jones model is often used with a cutoff radius of . See Mastny and de Pablo (Ref. 2) fa an analysis of the effect of this cutoff on the melting line.
Related potential models
It is relatively common the use of potential functions given by:
with and being positive integer numbers and , and is chosen to get the minimum value of being . Such forms are usually referred to as m-n Lennard-Jones Potential. The 9-3 Lennard-Jones interaction potential is often use to model the interaction between the atoms/molecules of a fluid and a continuous solid wall. On the '9-3 Lennard-Jones potential' page a justification of this use is presented.
Other dimensions
- 1-dimensional case: Lennard-Jones rods.
- 2-dimensional case: Lennard-Jones disks.
See also
- Phase diagram of the Lennard-Jones model
- Lennard-Jones model: virial coefficients
- Lennard-Jones equation of state
- Lennard-Jones sticks
References
- J. E. Lennard-Jones, "Cohesion", Proceedings of the Physical Society, 43 pp. 461-482 (1931)
- Ethan A. Mastny and Juan J. de Pablo "Melting line of the Lennard-Jones system, infinite size, and full potential", Journal of Chemical Physics 127 104504 (2007)
- J. M. Caillol " Critical-point of the Lennard-Jones fluid: A finite-size scaling study", Journal of Chemical Physics 109 pp. 4885-4893 (1998)
![{\displaystyle \Phi (r)=4\epsilon \left[\left({\frac {\sigma }{r}}\right)^{12}-\left({\frac {\sigma }{r}}\right)^{6}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b0ecd4ec335b5149b5f30ba2d02a6049d0407207)



















![{\displaystyle \Phi (r)=c_{m,n}\epsilon \left[\left({\frac {\sigma }{r}}\right)^{m}-\left({\frac {\sigma }{r}}\right)^{n}\right].}](https://wikimedia.org/api/rest_v1/media/math/render/svg/58317a77137d2a636f30e2a4a48c2ba87b516598)




