Hard sphere model: Difference between revisions
Carl McBride (talk | contribs) m (Undo revision 11738 by 158.64.77.126 (talk)) |
(Fixed broken links in ref. 5) |
||
| (22 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
[[Image:sphere_green.png|thumb|right]] | [[Image:sphere_green.png|thumb|right]] | ||
[[Image:Hard-sphere phase diagram pressure vs packing fraction.png|thumb|right|Phase diagram (pressure vs packing fraction) of hard sphere system (Solid line - stable branch, dashed line - metastable branch)]] | |||
The '''hard sphere model''' (sometimes known as the ''rigid sphere model'') is defined as | The '''hard sphere model''' (sometimes known as the ''rigid sphere model'') is defined as | ||
| Line 17: | Line 18: | ||
<ref>[http://dx.doi.org/10.1063/1.1743957 B. J. Alder and T. E. Wainwright "Phase Transition for a Hard Sphere System", Journal of Chemical Physics '''27''' pp. 1208-1209 (1957)]</ref>, much of this work undertaken at the Los Alamos Scientific Laboratory on the world's first electronic digital computer ENIAC <ref>[http://ftp.arl.army.mil/~mike/comphist/eniac-story.html The ENIAC Story]</ref>. | <ref>[http://dx.doi.org/10.1063/1.1743957 B. J. Alder and T. E. Wainwright "Phase Transition for a Hard Sphere System", Journal of Chemical Physics '''27''' pp. 1208-1209 (1957)]</ref>, much of this work undertaken at the Los Alamos Scientific Laboratory on the world's first electronic digital computer ENIAC <ref>[http://ftp.arl.army.mil/~mike/comphist/eniac-story.html The ENIAC Story]</ref>. | ||
==Liquid phase radial distribution function== | ==Liquid phase radial distribution function== | ||
The following are a series of plots of the hard sphere [[radial distribution function]] <ref>The [[total correlation function]] data was produced using the [ | The following are a series of plots of the hard sphere [[radial distribution function]] <ref>The [[total correlation function]] data was produced using the [https://old.vscht.cz/fch/software/hsmd/hspline-8-2004.zip computer code] written by [https://web.vscht.cz/~kolafaj/ Jiří Kolafa]</ref> shown for different values of the number density <math>\rho</math>. The horizontal axis is in units of <math>\sigma</math> where <math>\sigma</math> is set to be 1. Click on image of interest to see a larger view. | ||
:{| border="1" | :{| border="1" | ||
|- | |- | ||
| Line 30: | Line 31: | ||
where the [[second virial coefficient]], <math>B_2</math>, is given by | where the [[second virial coefficient]], <math>B_2</math>, is given by | ||
:<math>B_2 = \frac{2\pi}{3}\sigma^3</math>. | :<math>B_2 = \frac{2\pi}{3}\sigma^3</math>. | ||
Carnahan and Starling <ref>[http://dx.doi.org/10.1063/1.1672048 N. F.Carnahan and K. E.Starling,"Equation of State for Nonattracting Rigid Spheres" Journal of Chemical Physics '''51''' pp. 635-636 (1969)]</ref> provided the following expression for <math>{\mathrm g}(\sigma^+)</math> (Eq. 3 in <ref name="Tao1" > </ref>) | Carnahan and Starling <ref>[http://dx.doi.org/10.1063/1.1672048 N. F.Carnahan and K. E.Starling,"Equation of State for Nonattracting Rigid Spheres" Journal of Chemical Physics '''51''' pp. 635-636 (1969)]</ref> provided the following expression for <math>{\mathrm g}(\sigma^+)</math> (Eq. 3 in <ref name="Tao1" ></ref>) | ||
:<math>{\mathrm g}(\sigma^+)= \frac{1-\eta/2}{(1-\eta)^3}</math> | :<math>{\mathrm g}(\sigma^+)= \frac{1-\eta/2}{(1-\eta)^3}</math> | ||
where <math>\eta</math> is the [[packing fraction]]. | where <math>\eta</math> is the [[packing fraction]]. | ||
| Line 47: | Line 48: | ||
<ref>[http://dx.doi.org/10.1063/1.2201699 M. López de Haro, A. Santos and S. B. Yuste "On the radial distribution function of a hard-sphere fluid", Journal of Chemical Physics '''124''' 236102 (2006)]</ref> | <ref>[http://dx.doi.org/10.1063/1.2201699 M. López de Haro, A. Santos and S. B. Yuste "On the radial distribution function of a hard-sphere fluid", Journal of Chemical Physics '''124''' 236102 (2006)]</ref> | ||
==Liquid-solid transition== | ==Liquid-solid transition== | ||
The hard sphere system undergoes a [[Solid-liquid phase transitions |liquid-solid]] [[First-order transitions |first order transition]] <ref name="HooverRee">[http://dx.doi.org/10.1063/1.1670641 William G. Hoover and Francis H. Ree "Melting Transition and Communal Entropy for Hard Spheres", Journal of Chemical Physics '''49''' pp. 3609-3617 (1968)]</ref>, sometimes referred to as the Kirkwood-Alder transition <ref name="GastRussel">[http://dx.doi.org/10.1063/1.882495 Alice P. Gast and William B. Russel "Simple Ordering in Complex Fluids", Physics Today '''51''' (12) pp. 24-30 (1998)]</ref>. | The hard sphere system undergoes a [[Solid-liquid phase transitions |liquid-solid]] [[First-order transitions |first order transition]] <ref name="HooverRee">[http://dx.doi.org/10.1063/1.1670641 William G. Hoover and Francis H. Ree "Melting Transition and Communal Entropy for Hard Spheres", Journal of Chemical Physics '''49''' pp. 3609-3617 (1968)]</ref> | ||
The liquid-solid coexistence densities (<math>\rho^* = \rho \sigma^3</math>) | <ref>[http://dx.doi.org/10.1063/1.4870524 Miguel Robles, Mariano López de Haro and Andrés Santos "Note: Equation of state and the freezing point in the hard-sphere model", Journal of Chemical Physics '''140''' 136101 (2014)]</ref>, sometimes referred to as the Kirkwood-Alder transition <ref name="GastRussel">[http://dx.doi.org/10.1063/1.882495 Alice P. Gast and William B. Russel "Simple Ordering in Complex Fluids", Physics Today '''51''' (12) pp. 24-30 (1998)]</ref>. | ||
The liquid-solid coexistence densities (<math>\rho^* = \rho \sigma^3=6\eta/\pi</math>) has been calculated to be | |||
:{| border="1" | :{| border="1" | ||
|- | |- | ||
| <math>\rho^*_{\mathrm {solid}}</math> || <math>\rho^*_{\mathrm {liquid}}</math> || Reference | | <math>\rho^*_{\mathrm {solid}}</math> || <math>\rho^*_{\mathrm {liquid}}</math> || Reference | ||
|- | |- | ||
| 1.041|| 0. | | 1.041(4)|| 0.943(4) || <ref name="HooverRee"></ref> | ||
|- | |- | ||
| 1.0376|| 0.9391 || <ref name="FrenkelSmitBook">Daan Frenkel and Berend Smit "Understanding Molecular Simulation: From Algorithms to Applications", Second Edition (2002) (ISBN 0-12-267351-4) p. 261.</ref> | | 1.0376|| 0.9391 || <ref name="FrenkelSmitBook">Daan Frenkel and Berend Smit "Understanding Molecular Simulation: From Algorithms to Applications", Second Edition (2002) (ISBN 0-12-267351-4) p. 261.</ref> | ||
| Line 65: | Line 67: | ||
| 1.037 || 0.938 || <ref>[http://dx.doi.org/10.1063/1.476396 Ruslan L. Davidchack and Brian B. Laird "Simulation of the hard-sphere crystal–melt interface", Journal of Chemical Physics '''108''' pp. 9452-9462 (1998)]</ref> | | 1.037 || 0.938 || <ref>[http://dx.doi.org/10.1063/1.476396 Ruslan L. Davidchack and Brian B. Laird "Simulation of the hard-sphere crystal–melt interface", Journal of Chemical Physics '''108''' pp. 9452-9462 (1998)]</ref> | ||
|- | |- | ||
| 1. | | 1.033(3) || 0.935(2) || <ref name="Miguel"> [http://dx.doi.org/10.1063/1.3023062 Enrique de Miguel "Estimating errors in free energy calculations from thermodynamic integration using fitted data", Journal of Chemical Physics '''129''' 214112 (2008)]</ref> | ||
|- | |||
| 1.03715(9) || 0.93890(7) || <ref name="MoirEtAl2021"> [https://doi.org/10.1063/5.0058892 Craig Moir, Leo Lue, and Marcus N. Bannerman "Tethered-particle model: The calculation of free energies for hard-sphere systems", Journal of Chemical Physics '''155''' 064504 (2021)]</ref> | |||
|} | |} | ||
The coexistence [[pressure]] | The coexistence [[pressure]] has been calculated to be | ||
:{| border="1" | :{| border="1" | ||
|- | |- | ||
| <math>p (k_BT/\sigma^3) </math> || Reference | | <math>p (k_BT/\sigma^3) </math> || Reference | ||
|- | |- | ||
| 11.567|| <ref name="FrenkelSmitBook"> </ref> | | 11.5727(10)|| <ref name="FernandezUCM">[http://dx.doi.org/10.1103/PhysRevLett.108.165701 L. A. Fernández, V. Martín-Mayor, B. Seoane, and P. Verrocchio "Equilibrium Fluid-Solid Coexistence of Hard Spheres", Physical Review Letters '''108''' 165701 (2012)]</ref> | ||
|- | |||
| 11.57(10) || <ref name="Fortini"></ref> | |||
|- | |||
| 11.567|| <ref name="FrenkelSmitBook"></ref> | |||
|- | |- | ||
| 11. | | 11.55(11) || <ref>[http://dx.doi.org/10.1088/0953-8984/9/41/006 Robin J. Speedy "Pressure of the metastable hard-sphere fluid", Journal of Physics: Condensed Matter '''9''' pp. 8591-8599 (1997)]</ref> | ||
|- | |- | ||
| 11.54(4) || <ref name="Noya"> </ref> | | 11.54(4) || <ref name="Noya"></ref> | ||
|- | |- | ||
| 11.50(9) || <ref>[http://dx.doi.org/10.1103/PhysRevLett.85.5138 N. B. Wilding and A. D. Bruce "Freezing by Monte Carlo Phase Switch", Physical Review Letters '''85''' pp. 5138-5141 (2000)]</ref> | | 11.50(9) || <ref>[http://dx.doi.org/10.1103/PhysRevLett.85.5138 N. B. Wilding and A. D. Bruce "Freezing by Monte Carlo Phase Switch", Physical Review Letters '''85''' pp. 5138-5141 (2000)]</ref> | ||
|- | |- | ||
| 11. | | 11.48(11) || <ref name="Miguel"></ref> | ||
|- | |- | ||
| 11. | | 11.43(17) || <ref>[http://dx.doi.org/10.1063/1.3244562 G. Odriozola "Replica exchange Monte Carlo applied to hard spheres", Journal of Chemical Physics '''131''' 144107 (2009)]</ref> | ||
|- | |- | ||
| 11. | | 11.550(4) || <ref name="MoirEtAl2021"></ref> | ||
|} | |} | ||
The coexistence [[chemical potential]] | The coexistence [[chemical potential]] has been calculated to be | ||
:{| border="1" | :{| border="1" | ||
|- | |- | ||
| <math>\mu (k_BT) </math> || Reference | | <math>\mu (k_BT) </math> || Reference | ||
|- | |- | ||
| 15.980(11) || <ref name="Miguel"> </ref> | | 15.980(11) || <ref name="Miguel"></ref> | ||
|- | |||
| 16.053(4) || <ref name="MoirEtAl2021"></ref> | |||
|} | |} | ||
The [[Helmholtz energy function]] (in units of <math>Nk_BT</math>) is given by | The [[Helmholtz energy function]] (in units of <math>Nk_BT</math>) is given by | ||
| Line 98: | Line 108: | ||
| <math>A_{\mathrm {solid}}</math> || <math>A_{\mathrm {liquid}}</math> || Reference | | <math>A_{\mathrm {solid}}</math> || <math>A_{\mathrm {liquid}}</math> || Reference | ||
|- | |- | ||
| 4.887(3) || 3.719(8) || <ref name="Miguel"> </ref> | | 4.887(3) || 3.719(8) || <ref name="Miguel"></ref> | ||
|} | |} | ||
The melting and crystallization process has been studied by Isobe and Krauth <ref>[http://dx.doi.org/10.1063/1.4929529 Masaharu Isobe and Werner Krauth "Hard-sphere melting and crystallization with event-chain Monte Carlo", Journal of Chemical Physics '''143''' 084509 (2015)]</ref>. | |||
==Helmholtz energy function== | ==Helmholtz energy function== | ||
| Line 107: | Line 119: | ||
| <math>\rho^*</math> || <math>A/(Nk_BT)</math>|| Reference | | <math>\rho^*</math> || <math>A/(Nk_BT)</math>|| Reference | ||
|- | |- | ||
| 0.25 || | | 0.25 || −1.766 <math>\pm</math> 0.002 || Table I <ref name="Schilling"> [http://dx.doi.org/10.1063/1.3274951 T. Schilling and F. Schmid "Computing absolute free energies of disordered structures by molecular simulation", Journal of Chemical Physics '''131''' 231102 (2009)]</ref> | ||
|- | |- | ||
| 0.50 || | | 0.50 || −0.152 <math>\pm</math> 0.002 || Table I <ref name="Schilling"></ref> | ||
|- | |- | ||
| 0.75 || | | 0.75 || 1.721 <math>\pm</math> 0.002 || Table I <ref name="Schilling"></ref> | ||
|- | |- | ||
| 1.04086 || 4.959 || Table VI <ref name="VegaNoya"> </ref> | | 1.04086 || 4.959 || Table VI <ref name="VegaNoya"></ref> | ||
|- | |- | ||
| 1.099975 || 5.631 || Table VI <ref name="VegaNoya"> </ref> | | 1.099975 || 5.631 || Table VI <ref name="VegaNoya"></ref> | ||
|- | |- | ||
| 1.150000 || 6.274 || Table VI <ref name="VegaNoya"> </ref> | | 1.150000 || 6.274 || Table VI <ref name="VegaNoya"></ref> | ||
|} | |} | ||
In <ref name="Schilling"></ref> the free energies are given without the ideal gas contribution <math>\ln(\rho^*)-1</math> . Hence, it was added to the free energies in the table. | |||
==Interfacial Helmholtz energy function== | ==Interfacial Helmholtz energy function== | ||
The [[Helmholtz energy function]] of the solid–liquid [[interface]] has been calculated using the [[cleaving method]] giving (Ref. <ref>[http://dx.doi.org/10.1063/1.3514144 Ruslan L. Davidchack "Hard spheres revisited: Accurate calculation of the solid–liquid interfacial free energy", Journal of Chemical Physics '''133''' 234701 (2010)]</ref> Table I): | The [[Helmholtz energy function]] of the solid–liquid [[interface]] has been calculated using the [[cleaving method]] giving (Ref. <ref>[http://dx.doi.org/10.1063/1.3514144 Ruslan L. Davidchack "Hard spheres revisited: Accurate calculation of the solid–liquid interfacial free energy", Journal of Chemical Physics '''133''' 234701 (2010)]</ref> Table I): | ||
| Line 125: | Line 140: | ||
| || [[work]] per unit area/<math>(k_BT/\sigma^2)</math> | | || [[work]] per unit area/<math>(k_BT/\sigma^2)</math> | ||
|- | |- | ||
| <math>\gamma_{100}</math> || 0.5820(19) | | <math>\gamma_{\{100\}}</math> || 0.5820(19) | ||
|- | |- | ||
| <math>\gamma_{ | | <math>\gamma_{\{100\}}</math> || 0.636(11) <ref name="FernandezUCM"></ref> | ||
|- | |- | ||
| <math>\gamma_{ | | <math>\gamma_{\{110\}}</math> || 0.5590(20) | ||
|- | |- | ||
| <math>\gamma_{120}</math> || 0.5669(20) | | <math>\gamma_{\{111\}}</math> || 0.5416(31) | ||
|- | |||
| <math>\gamma_{\{120\}}</math> || 0.5669(20) | |||
|} | |} | ||
==Solid structure== | ==Solid structure== | ||
The [http://mathworld.wolfram.com/KeplerConjecture.html Kepler conjecture] states that the optimal packing for three dimensional spheres is either cubic or hexagonal close [[Lattice Structures | packing]], both of which have maximum densities of <math>\pi/(3 \sqrt{2}) \approx 74.048%</math> <ref>[http://dx.doi.org/10.1038/26609 Neil J. A. Sloane "Kepler's conjecture confirmed", Nature '''395''' pp. 435-436 (1998)]</ref> | The [http://mathworld.wolfram.com/KeplerConjecture.html Kepler conjecture] states that the optimal packing for three dimensional spheres is either cubic or hexagonal close [[Lattice Structures | packing]], both of which have maximum densities of <math>\pi/(3 \sqrt{2}) \approx 74.048%</math><ref>[http://dx.doi.org/10.1038/26609 Neil J. A. Sloane "Kepler's conjecture confirmed", Nature '''395''' pp. 435-436 (1998)]</ref> | ||
<ref>[https://www.newscientist.com/article/dn26041-proof-confirmed-of-400-year-old-fruit-stacking-problem/ Jacob Aron "Proof confirmed of 400-year-old fruit-stacking problem", New Scientist daily news 12 August (2014)]</ref> | |||
<ref>[http://dx.doi.org/10.1103/PhysRevE.52.3632 C. F. Tejero, M. S. Ripoll, and A. Pérez "Pressure of the hard-sphere solid", Physical Review E '''52''' pp. 3632-3636 (1995)]</ref>. However, for hard spheres at close packing the [[Building up a face centered cubic lattice |face centred cubic]] phase is the more stable | <ref>[http://dx.doi.org/10.1103/PhysRevE.52.3632 C. F. Tejero, M. S. Ripoll, and A. Pérez "Pressure of the hard-sphere solid", Physical Review E '''52''' pp. 3632-3636 (1995)]</ref>. However, for hard spheres at close packing the [[Building up a face centered cubic lattice |face centred cubic]] phase is the more stable | ||
<ref>[http://dx.doi.org/10.1039/a701761h Leslie V. Woodcock "Computation of the free energy for alternative crystal structures of hard spheres", Faraday Discussions '''106''' pp. 325 - 338 (1997)]</ref> | <ref>[http://dx.doi.org/10.1039/a701761h Leslie V. Woodcock "Computation of the free energy for alternative crystal structures of hard spheres", Faraday Discussions '''106''' pp. 325-338 (1997)]</ref>, with a [[Helmholtz energy function]] difference in the [[thermodynamic limit]] between the hexagonal close packed and face centered cubic crystals at close packing of 0.001164(8) <math>Nk_BT</math><ref>[http://dx.doi.org/10.1080/00268976.2014.982736 Eva G. Noya and Noé G. Almarza "Entropy of hard spheres in the close-packing limit", Molecular Physics '''113''' pp. 1061-1068 (2015)]</ref>. Recently evidence has been found for a metastable cI16 phase <ref>[https://doi.org/10.1063/1.5009099 Vadim B. Warshavsky, David M. Ford, and Peter A. Monson "On the mechanical stability of the body-centered cubic phase and the emergence of a metastable cI16 phase in classical hard sphere solids", Journal of Chemical Physics '''148''' 024502 (2018)]</ref> indicating the ''"cI16 is a mechanically stable structure that can spontaneously emerge from a bcc starting point but it is thermodynamically metastable relative to fcc or hcp".'' | ||
*See also: [[Equations of state for crystals of hard spheres]] | *See also: [[Equations of state for crystals of hard spheres]] | ||
==Direct correlation function== | ==Direct correlation function== | ||
For the [[direct correlation function]] see: | For the [[direct correlation function]] see: | ||
Latest revision as of 12:47, 23 May 2023
The hard sphere model (sometimes known as the rigid sphere model) is defined as
where is the intermolecular pair potential between two spheres at a distance , and is the diameter of the sphere. The hard sphere model can be considered to be a special case of the hard ellipsoid model, where each of the semi-axes has the same length, .
First simulations of hard spheres (1954-1957)[edit]
The hard sphere model, along with its two-dimensional manifestation hard disks, was one of the first ever systems studied using computer simulation techniques with a view to understanding the thermodynamics of the liquid and solid phases and their corresponding phase transition [1] [2] [3], much of this work undertaken at the Los Alamos Scientific Laboratory on the world's first electronic digital computer ENIAC [4].
Liquid phase radial distribution function[edit]
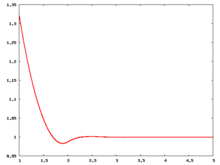
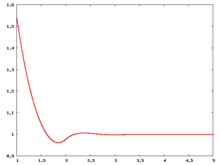
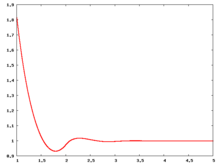
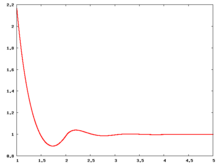
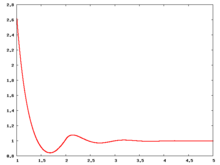
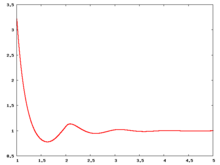
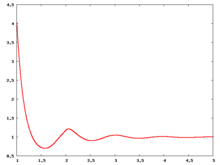
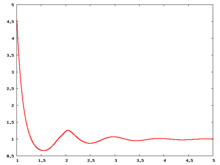
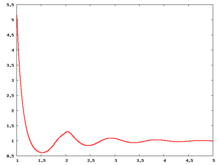
The following are a series of plots of the hard sphere radial distribution function [5] shown for different values of the number density . The horizontal axis is in units of where is set to be 1. Click on image of interest to see a larger view.
The value of the radial distribution at contact, , can be used to calculate the pressure via the equation of state (Eq. 1 in [6])
where the second virial coefficient, , is given by
- .
Carnahan and Starling [7] provided the following expression for (Eq. 3 in [6])
where is the packing fraction.
Over the years many groups have studied the radial distribution function of the hard sphere model: [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18]
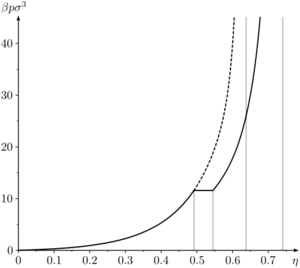
Liquid-solid transition[edit]
The hard sphere system undergoes a liquid-solid first order transition [19] [20], sometimes referred to as the Kirkwood-Alder transition [21]. The liquid-solid coexistence densities () has been calculated to be
Reference 1.041(4) 0.943(4) [19] 1.0376 0.9391 [22] 1.0367(10) 0.9387(10) [23] 1.0372 0.9387 [24] 1.0369(33) 0.9375(14) [25] 1.037 0.938 [26] 1.033(3) 0.935(2) [27] 1.03715(9) 0.93890(7) [28]
The coexistence pressure has been calculated to be
Reference 11.5727(10) [29] 11.57(10) [23] 11.567 [22] 11.55(11) [30] 11.54(4) [25] 11.50(9) [31] 11.48(11) [27] 11.43(17) [32] 11.550(4) [28]
The coexistence chemical potential has been calculated to be
The Helmholtz energy function (in units of ) is given by
Reference 4.887(3) 3.719(8) [27]
The melting and crystallization process has been studied by Isobe and Krauth [33].
Helmholtz energy function[edit]
Values for the Helmholtz energy function () are given in the following Table:
Reference 0.25 −1.766 0.002 Table I [34] 0.50 −0.152 0.002 Table I [34] 0.75 1.721 0.002 Table I [34] 1.04086 4.959 Table VI [24] 1.099975 5.631 Table VI [24] 1.150000 6.274 Table VI [24]
In [34] the free energies are given without the ideal gas contribution . Hence, it was added to the free energies in the table.
Interfacial Helmholtz energy function[edit]
The Helmholtz energy function of the solid–liquid interface has been calculated using the cleaving method giving (Ref. [35] Table I):
Solid structure[edit]
The Kepler conjecture states that the optimal packing for three dimensional spheres is either cubic or hexagonal close packing, both of which have maximum densities of [36] [37] [38]. However, for hard spheres at close packing the face centred cubic phase is the more stable [39], with a Helmholtz energy function difference in the thermodynamic limit between the hexagonal close packed and face centered cubic crystals at close packing of 0.001164(8) [40]. Recently evidence has been found for a metastable cI16 phase [41] indicating the "cI16 is a mechanically stable structure that can spontaneously emerge from a bcc starting point but it is thermodynamically metastable relative to fcc or hcp".
Direct correlation function[edit]
For the direct correlation function see: [42] [43]
Bridge function[edit]
Details of the bridge function for hard sphere can be found in the following publication [44]
Equations of state[edit]
- Main article: Equations of state for hard spheres
Virial coefficients[edit]
- Main article: Hard sphere: virial coefficients
Experimental results[edit]
Pusey and van Megen used a suspension of PMMA particles of radius 305 10 nm, suspended in poly-12-hydroxystearic acid [45] For results obtained from the Colloidal Disorder - Order Transition (CDOT) experiments performed on-board the Space Shuttles Columbia and Discovery see Ref. [46]
Mixtures[edit]
Related systems[edit]
Hard spheres in other dimensions:
- 1-dimensional case: hard rods.
- 2-dimensional case: hard disks.
- Hard hyperspheres
References[edit]
- ↑ Marshall N. Rosenbluth and Arianna W. Rosenbluth "Further Results on Monte Carlo Equations of State", Journal of Chemical Physics 22 pp. 881-884 (1954)
- ↑ W. W. Wood and J. D. Jacobson "Preliminary Results from a Recalculation of the Monte Carlo Equation of State of Hard Spheres", Journal of Chemical Physics 27 pp. 1207-1208 (1957)
- ↑ B. J. Alder and T. E. Wainwright "Phase Transition for a Hard Sphere System", Journal of Chemical Physics 27 pp. 1208-1209 (1957)
- ↑ The ENIAC Story
- ↑ The total correlation function data was produced using the computer code written by Jiří Kolafa
- ↑ 6.0 6.1 Fu-Ming Tao, Yuhua Song, and E. A. Mason "Derivative of the hard-sphere radial distribution function at contact", Physical Review A 46 pp. 8007-8008 (1992)
- ↑ N. F.Carnahan and K. E.Starling,"Equation of State for Nonattracting Rigid Spheres" Journal of Chemical Physics 51 pp. 635-636 (1969)
- ↑ John G. Kirkwood, Eugene K. Maun, and Berni J. Alder "Radial Distribution Functions and the Equation of State of a Fluid Composed of Rigid Spherical Molecules", Journal of Chemical Physics 18 pp. 1040- (1950)
- ↑ B. R. A. Nijboer and L. Van Hove "Radial Distribution Function of a Gas of Hard Spheres and the Superposition Approximation", Physical Review 85 pp. 777 - 783 (1952)
- ↑ B. J. Alder, S. P. Frankel, and V. A. Lewinson "Radial Distribution Function Calculated by the Monte-Carlo Method for a Hard Sphere Fluid", Journal of Chemical Physics 23 pp. 417- (1955)
- ↑ Francis H. Ree, R. Norris Keeler, and Shaun L. McCarthy "Radial Distribution Function of Hard Spheres", Journal of Chemical Physics 44 pp. 3407- (1966)
- ↑ W. R. Smith and D. Henderson "Analytical representation of the Percus-Yevick hard-sphere radial distribution function", Molecular Physics 19 pp. 411-415 (1970)
- ↑ J. A. Barker and D. Henderson "Monte Carlo values for the radial distribution function of a system of fluid hard spheres", Molecular Physics 21 pp. 187-191 (1971)
- ↑ J. M. Kincaid and J. J. Weis "Radial distribution function of a hard-sphere solid", Molecular Physics 34 pp. 931-938 (1977)
- ↑ S. Bravo Yuste and A. Santos "Radial distribution function for hard spheres", Physical Review A 43 pp. 5418-5423 (1991)
- ↑ Jaeeon Chang and Stanley I. Sandler "A real function representation for the structure of the hard-sphere fluid", Molecular Physics 81 pp. 735-744 (1994)
- ↑ Andrij Trokhymchuk, Ivo Nezbeda and Jan Jirsák "Hard-sphere radial distribution function again", Journal of Chemical Physics 123 024501 (2005)
- ↑ M. López de Haro, A. Santos and S. B. Yuste "On the radial distribution function of a hard-sphere fluid", Journal of Chemical Physics 124 236102 (2006)
- ↑ 19.0 19.1 William G. Hoover and Francis H. Ree "Melting Transition and Communal Entropy for Hard Spheres", Journal of Chemical Physics 49 pp. 3609-3617 (1968)
- ↑ Miguel Robles, Mariano López de Haro and Andrés Santos "Note: Equation of state and the freezing point in the hard-sphere model", Journal of Chemical Physics 140 136101 (2014)
- ↑ Alice P. Gast and William B. Russel "Simple Ordering in Complex Fluids", Physics Today 51 (12) pp. 24-30 (1998)
- ↑ 22.0 22.1 Daan Frenkel and Berend Smit "Understanding Molecular Simulation: From Algorithms to Applications", Second Edition (2002) (ISBN 0-12-267351-4) p. 261.
- ↑ 23.0 23.1 Andrea Fortini and Marjolein Dijkstra "Phase behaviour of hard spheres confined between parallel hard plates: manipulation of colloidal crystal structures by confinement", Journal of Physics: Condensed Matter 18 pp. L371-L378 (2006)
- ↑ 24.0 24.1 24.2 24.3 Carlos Vega and Eva G. Noya "Revisiting the Frenkel-Ladd method to compute the free energy of solids: The Einstein molecule approach", Journal of Chemical Physics 127 154113 (2007)
- ↑ 25.0 25.1 Eva G. Noya, Carlos Vega, and Enrique de Miguel "Determination of the melting point of hard spheres from direct coexistence simulation methods", Journal of Chemical Physics 128 154507 (2008)
- ↑ Ruslan L. Davidchack and Brian B. Laird "Simulation of the hard-sphere crystal–melt interface", Journal of Chemical Physics 108 pp. 9452-9462 (1998)
- ↑ 27.0 27.1 27.2 27.3 Enrique de Miguel "Estimating errors in free energy calculations from thermodynamic integration using fitted data", Journal of Chemical Physics 129 214112 (2008)
- ↑ 28.0 28.1 28.2 Craig Moir, Leo Lue, and Marcus N. Bannerman "Tethered-particle model: The calculation of free energies for hard-sphere systems", Journal of Chemical Physics 155 064504 (2021)
- ↑ 29.0 29.1 L. A. Fernández, V. Martín-Mayor, B. Seoane, and P. Verrocchio "Equilibrium Fluid-Solid Coexistence of Hard Spheres", Physical Review Letters 108 165701 (2012)
- ↑ Robin J. Speedy "Pressure of the metastable hard-sphere fluid", Journal of Physics: Condensed Matter 9 pp. 8591-8599 (1997)
- ↑ N. B. Wilding and A. D. Bruce "Freezing by Monte Carlo Phase Switch", Physical Review Letters 85 pp. 5138-5141 (2000)
- ↑ G. Odriozola "Replica exchange Monte Carlo applied to hard spheres", Journal of Chemical Physics 131 144107 (2009)
- ↑ Masaharu Isobe and Werner Krauth "Hard-sphere melting and crystallization with event-chain Monte Carlo", Journal of Chemical Physics 143 084509 (2015)
- ↑ 34.0 34.1 34.2 34.3 T. Schilling and F. Schmid "Computing absolute free energies of disordered structures by molecular simulation", Journal of Chemical Physics 131 231102 (2009)
- ↑ Ruslan L. Davidchack "Hard spheres revisited: Accurate calculation of the solid–liquid interfacial free energy", Journal of Chemical Physics 133 234701 (2010)
- ↑ Neil J. A. Sloane "Kepler's conjecture confirmed", Nature 395 pp. 435-436 (1998)
- ↑ Jacob Aron "Proof confirmed of 400-year-old fruit-stacking problem", New Scientist daily news 12 August (2014)
- ↑ C. F. Tejero, M. S. Ripoll, and A. Pérez "Pressure of the hard-sphere solid", Physical Review E 52 pp. 3632-3636 (1995)
- ↑ Leslie V. Woodcock "Computation of the free energy for alternative crystal structures of hard spheres", Faraday Discussions 106 pp. 325-338 (1997)
- ↑ Eva G. Noya and Noé G. Almarza "Entropy of hard spheres in the close-packing limit", Molecular Physics 113 pp. 1061-1068 (2015)
- ↑ Vadim B. Warshavsky, David M. Ford, and Peter A. Monson "On the mechanical stability of the body-centered cubic phase and the emergence of a metastable cI16 phase in classical hard sphere solids", Journal of Chemical Physics 148 024502 (2018)
- ↑ C. F. Tejero and M. López De Haro "Direct correlation function of the hard-sphere fluid", Molecular Physics 105 pp. 2999-3004 (2007)
- ↑ Matthew Dennison, Andrew J. Masters, David L. Cheung, and Michael P. Allen "Calculation of direct correlation function for hard particles using a virial expansion", Molecular Physics pp. 375-382 (2009)
- ↑ Jiri Kolafa, Stanislav Labik and Anatol Malijevsky "The bridge function of hard spheres by direct inversion of computer simulation data", Molecular Physics 100 pp. 2629-2640 (2002)
- ↑ P. N. Pusey and W. van Megen "Phase behaviour of concentrated suspensions of nearly hard colloidal spheres", Nature 320 pp. 340-342 (1986)
- ↑ Z. Chenga, P. M. Chaikina, W. B. Russelb, W. V. Meyerc, J. Zhub, R. B. Rogersc and R. H. Ottewilld, "Phase diagram of hard spheres", Materials & Design 22 pp. 529-534 (2001)
Related reading
- "Theory and Simulation of Hard-Sphere Fluids and Related Systems", Lecture Notes in Physics 753/2008 Springer (2008)
- Laura Filion, Michiel Hermes, Ran Ni and Marjolein Dijkstra "Crystal nucleation of hard spheres using molecular dynamics, umbrella sampling, and forward flux sampling: A comparison of simulation techniques", Journal of Chemical Physics 133 244115 (2010)
External links[edit]
- Hard disks and spheres computer code on SMAC-wiki.