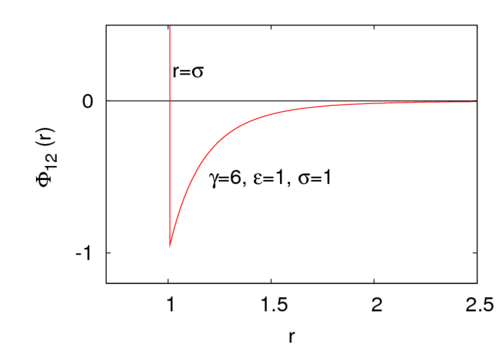Sutherland potential: Difference between revisions
Jump to navigation
Jump to search
Carl McBride (talk | contribs) (New page: The '''Sutherland potential''' is given by :<math> \Phi\left( r \right) = \left\{ \begin{array}{lll} \infty & ; & r \leq \sigma \\ - \left( \frac{ \epsilon \sigma }{r}\right)^{\gamma} & ...) |
Carl McBride (talk | contribs) m (Added a correct but broken (Taylor & Francis!) DOI.) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
The '''Sutherland potential''' is given by | The '''Sutherland potential''' <ref>[http://dx.doi.org/10.1080/14786449308620508 William Sutherland "The viscosity of gases and molecular force", Philosophical Magazine '''36''' pp. 507-531 (1893)]</ref><ref>[http://dx.doi.org/10.1103/RevModPhys.36.1025 H. W. Graben and R. D. Present "Third Virial Coefficient for the Sutherland (∞, ν) Potential", Reviews of Modern Physics '''36''' pp. 1025-1033 (1964)]</ref> is given by | ||
:<math> | :<math> | ||
\ | \Phi_{12}\left( r \right) = | ||
\left\{ \begin{array}{lll} | \left\{ \begin{array}{lll} | ||
\infty & ; & r \leq \sigma \\ | \infty & ; & r \leq \sigma \\ | ||
- \left( \frac{ | - \epsilon\left( \frac{ \sigma }{r}\right)^{\gamma} & ; & r > \sigma | ||
\end{array} \right. | \end{array} \right. | ||
</math> | </math> | ||
where <math> \ | where <math> \Phi_{12}\left( r \right) </math> is the [[intermolecular pair potential]], <math>r := |\mathbf{r}_1 - \mathbf{r}_2|</math> is the distance between site 1 and site 2, | ||
<math> \sigma </math> is the hard | <math> \sigma </math> is the radius of the central hard core, <math> \epsilon </math> is the energy well depth (<math> \epsilon > 0 </math>), and | ||
<math> \gamma </math> is a parameter that controls the interaction range. | <math> \gamma </math> is a parameter that controls the interaction range. For example, for <math>\gamma=6</math> the potential look like | ||
:[[Image:Sutherland.png|500px]] | |||
==References== | ==References== | ||
<references/> | |||
'''Related reading''' | |||
*[http://dx.doi.org/10.1063/1.431141 D. Levi and M. de Llano "Closed form of second virial coefficient for Sutherland potential", Journal of Chemical Physics '''63''' pp. 4561-4562 (1975)] | |||
*[http://dx.doi.org/10.1142/S0217979204025142 Jianxiang Tian and Yuanxing Gui "Liquid-gas phase transition to first order of an argon-like fluid modeled by the hard-core similar Sutherland potential", International Journal of Modern Physics B '''18''' pp. 2057-2069 (2004)] | |||
*[http://dx.doi.org/10.1016/j.fluid.2007.01.011 A. Díez, J. Largo and J. R. Solana "Structure and thermodynamic properties of Sutherland fluids from computer simulation and the Tang–Lu integral equation theory", Fluid Phase Equilibria '''253''' pp. 67-73 (2007)] | |||
*[http://dx.doi.org/10.1063/1.2828720 Jianguo Mi, Yiping Tang, and Chongli Zhong "Theoretical study of Sutherland fluids with long-range, short-range, and highly short-range potential parameters", Journal of Chemical Physics '''128''' 054503 (2008)] | |||
*[http://dx.doi.org/10.1063/1.3371710 Roman Melnyk, Pedro Orea, Ivo Nezbeda, and Andrij Trokhymchuk "Liquid/vapor coexistence and surface tension of the Sutherland fluid with a variable range of interaction: Computer simulation and perturbation theory studies", Journal of Chemical Physics '''132''' 134504 (2010)] | |||
*[http://dx.doi.org/10.1016/j.fluid.2009.10.027 F. Paragand, F. Feyzi and B. Behzadi "Application of the SAFT-VR equation of state to vapor–liquid equilibrium calculations for pure components and binary mixtures using the Sutherland potential", Fluid Phase Equilibria '''290''' pp. 181-194 (2010)] | |||
[[Category: Models]] | [[Category: Models]] | ||
Latest revision as of 15:32, 6 April 2010
The Sutherland potential [1][2] is given by
where is the intermolecular pair potential, is the distance between site 1 and site 2, is the radius of the central hard core, is the energy well depth (), and is a parameter that controls the interaction range. For example, for the potential look like
References[edit]
Related reading
- D. Levi and M. de Llano "Closed form of second virial coefficient for Sutherland potential", Journal of Chemical Physics 63 pp. 4561-4562 (1975)
- Jianxiang Tian and Yuanxing Gui "Liquid-gas phase transition to first order of an argon-like fluid modeled by the hard-core similar Sutherland potential", International Journal of Modern Physics B 18 pp. 2057-2069 (2004)
- A. Díez, J. Largo and J. R. Solana "Structure and thermodynamic properties of Sutherland fluids from computer simulation and the Tang–Lu integral equation theory", Fluid Phase Equilibria 253 pp. 67-73 (2007)
- Jianguo Mi, Yiping Tang, and Chongli Zhong "Theoretical study of Sutherland fluids with long-range, short-range, and highly short-range potential parameters", Journal of Chemical Physics 128 054503 (2008)
- Roman Melnyk, Pedro Orea, Ivo Nezbeda, and Andrij Trokhymchuk "Liquid/vapor coexistence and surface tension of the Sutherland fluid with a variable range of interaction: Computer simulation and perturbation theory studies", Journal of Chemical Physics 132 134504 (2010)
- F. Paragand, F. Feyzi and B. Behzadi "Application of the SAFT-VR equation of state to vapor–liquid equilibrium calculations for pure components and binary mixtures using the Sutherland potential", Fluid Phase Equilibria 290 pp. 181-194 (2010)