Van der Waals equation of state: Difference between revisions
Jfcastillo (talk | contribs) (small changes for consistency) |
Carl McBride (talk | contribs) m (Slight tidy.) |
||
| Line 12: | Line 12: | ||
* <math> T </math> is the absolute [[temperature]], | * <math> T </math> is the absolute [[temperature]], | ||
* <math> R </math> is the [[molar gas constant]]; <math> R = N_A k_B </math>, with <math> N_A </math> being the [[Avogadro constant]] and <math>k_B</math> being the [[Boltzmann constant]]. | * <math> R </math> is the [[molar gas constant]]; <math> R = N_A k_B </math>, with <math> N_A </math> being the [[Avogadro constant]] and <math>k_B</math> being the [[Boltzmann constant]]. | ||
==Critical point== | |||
At the [[Critical points |critical point]] one has <math>\left.\frac{\partial p}{\partial v}\right|_{T=T_c}=0 </math>, and <math>\left.\frac{\partial^2 p}{\partial v^2}\right|_{T=T_c}=0 </math>, leading to | |||
:<math> | :<math>T_c= \frac{8a}{27bR}</math> | ||
:<math>p_c=\frac{a}{27b^2}</math> | :<math>p_c=\frac{a}{27b^2}</math> | ||
:<math>\left.V_c\right.=3b</math>. | :<math>\left.V_c\right.=3b</math>. | ||
and | |||
:<math>\frac{p_cV_c}{T_c}= \frac{3R}{8}</math> | |||
which then leads to | |||
:<math>a= \frac{27}{64}\frac{R^2T_c^2}{P_c}</math> | |||
:<math>b= \frac{RT_c}{8P_c}</math> | |||
==Dimensionless formulation== | ==Dimensionless formulation== | ||
If one takes the following reduced quantities | If one takes the following reduced quantities | ||
Revision as of 13:00, 20 October 2009
The van der Waals equation of state, developed by Johannes Diderik van der Waals, takes into account two features that are absent in the ideal gas equation of state; the parameter Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle b } introduces somehow the repulsive behavior between pairs of molecules at short distances, it represents the minimum molar volume of the system, whereas Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle a } measures the attractive interactions between the molecules. The van der Waals equation of state leads to a liquid-vapor equilibrium at low temperatures, with the corresponding critical point.
Equation of state
The van der Waals equation of state can be written as
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \left. p = \frac{ n R T}{V - n b } - a \left( \frac{ n}{V} \right)^2 \right. } .
where:
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p } is the pressure,
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V } is the volume,
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle n } is the number of moles,
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T } is the absolute temperature,
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle R } is the molar gas constant; Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle R = N_A k_B } , with Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle N_A } being the Avogadro constant and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle k_B} being the Boltzmann constant.
Critical point
At the critical point one has Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \left.\frac{\partial p}{\partial v}\right|_{T=T_c}=0 } , and Failed to parse (Conversion error. Server ("https://wikimedia.org/api/rest_") reported: "Cannot get mml. Server problem."): {\displaystyle \left.{\frac {\partial ^{2}p}{\partial v^{2}}}\right|_{T=T_{c}}=0} , leading to
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T_c= \frac{8a}{27bR}}
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p_c=\frac{a}{27b^2}}
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \left.V_c\right.=3b} .
and
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \frac{p_cV_c}{T_c}= \frac{3R}{8}}
which then leads to
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle a= \frac{27}{64}\frac{R^2T_c^2}{P_c}}
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle b= \frac{RT_c}{8P_c}}
Dimensionless formulation
If one takes the following reduced quantities
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{p} = \frac{p}{p_c};~ \tilde{V} = \frac{V}{V_c}; ~\tilde{t} = \frac{T}{T_c};}
one arrives at
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{p} = \frac{8\tilde{t}}{3\tilde{V} -1} -\frac{3}{\tilde{V}^2}}
The following image is a plot of the isotherms Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T/T_c} = 0.85, 0.90, 0.95, 1.0 and 1.05 (from bottom to top) for the van der Waals equation of state:
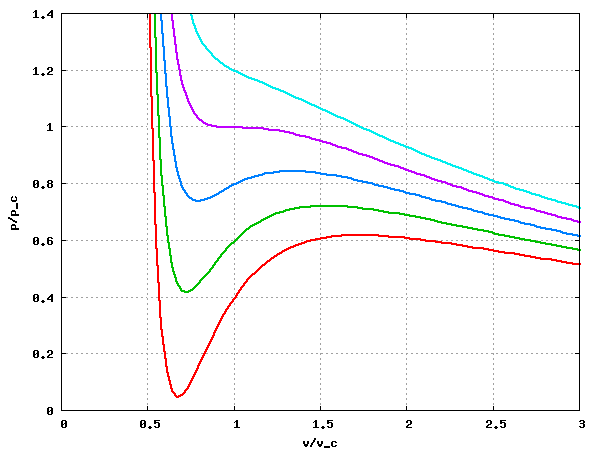
Maxwell's equal area construction
Interesting reading
- Johannes Diderik van der Waals "The Equation of State for Gases and Liquids", Nobel Lecture, December 12, 1910
- Luis Gonzalez MacDowell and Peter Virnau "El integrante lazo de van der Waals", Anales de la Real Sociedad Española de Química 101 #1 pp. 19-30 (2005)
References
- J. D. van der Waals "Over de Continuiteit van den Gas- en Vloeistoftoestand", doctoral thesis, Leiden, A,W, Sijthoff (1873).
English translation: