Van der Waals equation of state: Difference between revisions
Jump to navigation
Jump to search
Carl McBride (talk | contribs) No edit summary |
Carl McBride (talk | contribs) No edit summary |
||
| Line 29: | Line 29: | ||
and at | and at | ||
:<math>\left.v_c\right.=3b</math>. | :<math>\left.v_c\right.=3b</math>. | ||
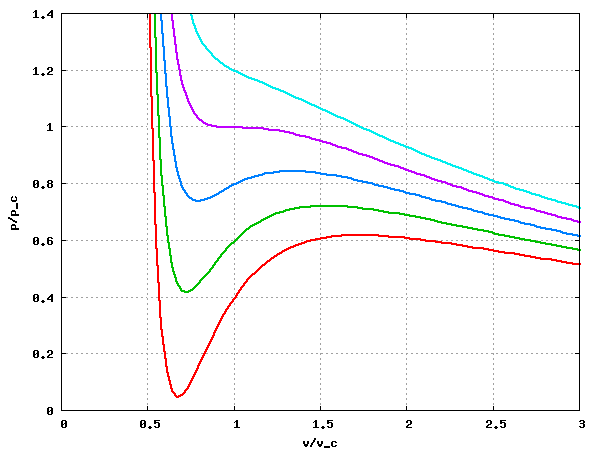
==Dimensionless formulation== | |||
If one takes the following quantities | |||
:<math>\tilde{p} = \frac{p}{p_c};~ \tilde{v} = \frac{v}{v_c}; ~\tilde{t} = \frac{T}{T_c};</math> | |||
one arrives at | |||
:<math>\tilde{p} = \frac{8\tilde{t}}{3\tilde{v} -1} -\frac{3}{\tilde{v}^2}</math> | |||
[[Image:vdW_isotherms.png|center|Plot of the isotherms T/T_c = 0.85, 0.90, 0.95, 1.0 and 1.05 for the Van der Waals equation of state]] | |||
==Interesting reading== | ==Interesting reading== | ||
*[http://store.doverpublications.com/0486495930.html J. D. van der Waals "On the Continuity of the Gaseous and Liquid States", Dover Publications ISBN: 0486495930] | *[http://store.doverpublications.com/0486495930.html J. D. van der Waals "On the Continuity of the Gaseous and Liquid States", Dover Publications ISBN: 0486495930] | ||
Revision as of 14:19, 24 September 2007
The van der Waals equation of state, developed by Johannes Diderik van der Waals, can be written as
- .
where:
- is the pressure
- is the volume
- is the number of moles
- is the absolute temperature
- is the Gas constant; , with being Avogadro constant
The van der Waals equation of state takes into account two features that are absent in the ideal Gas equation of state: The parameter introduces somehow the repulsive behavior between pairs of molecules at short distances, it represents the minimum molar volume of the system, whereas measures the attractive interactions between the molecules. The van der Waals equation of state leads to a liquid-vapor equilibrium at low temperatures, with the corresponding critical point.
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle a= \frac{27}{64}\frac{R^2T_c^2}{P_c}}
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle b= \frac{RT_c}{8P_c}}
Critical point
The critical point for the van der Waals equation of state can be found at
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle T_c= \frac{8a}{27bR}} ,
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p_c=\frac{a}{27b^2}}
and at
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \left.v_c\right.=3b} .
Dimensionless formulation
If one takes the following quantities
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{p} = \frac{p}{p_c};~ \tilde{v} = \frac{v}{v_c}; ~\tilde{t} = \frac{T}{T_c};}
one arrives at
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{p} = \frac{8\tilde{t}}{3\tilde{v} -1} -\frac{3}{\tilde{v}^2}}
Interesting reading
- J. D. van der Waals "On the Continuity of the Gaseous and Liquid States", Dover Publications ISBN: 0486495930
- Johannes Diderik van der Waals "The Equation of State for Gases and Liquids", Nobel Lecture, December 12, 1910
- Luis Gonzalez MacDowell and Peter Virnau "El integrante lazo de Van der Waals", Anales de la Real Sociedad Española de Química 101 #1 pp. 19-30 (2005)









