Uhlenbeck-Ford model: Difference between revisions
Carl McBride (talk | contribs) m (Added some internal links) |
|||
| Line 1: | Line 1: | ||
The '''Uhlenbeck-Ford model''' (UFM), originally named Gaussian gas <ref>D. McQuarrie, Statistical Mechanics (University Science Books, 2000).</ref>, was proposed by G. Uhlenbeck and G. Ford <ref name="UF">G. Uhlenbeck and G. Ford, in Studies in Statistical Mechanics— The Theory of Linear Graphs with Application to the Theory of the Virial Development of the Properties of Gases, edited by G. E. Uhlenbeck and J. de Boer (North-Holland, Amsterdam, 1962), Vol. 2. </ref> for the theoretical study of imperfect gases. This model is characterized by an ultrasoft, purely repulsive pairwise interaction potential that diverges logarithmically at the origin and features an energy scale that coincides with the thermal energy unit <math>k_B T</math> where <math>k_B</math> is the | The '''Uhlenbeck-Ford model''' (UFM), originally named Gaussian gas <ref>D. McQuarrie, Statistical Mechanics (University Science Books, 2000).</ref>, was proposed by G. Uhlenbeck and G. Ford <ref name="UF">G. Uhlenbeck and G. Ford, in Studies in Statistical Mechanics— The Theory of Linear Graphs with Application to the Theory of the Virial Development of the Properties of Gases, edited by G. E. Uhlenbeck and J. de Boer (North-Holland, Amsterdam, 1962), Vol. 2. </ref> for the theoretical study of imperfect gases. This model is characterized by an ultrasoft, purely repulsive pairwise interaction potential that diverges logarithmically at the origin and features an energy scale that coincides with the thermal energy unit <math>k_B T</math> where <math>k_B</math> is the [[Boltzmann constant]] and <math>T</math> the absolute [[temperature]]. The particular functional form of the potential permits, in principle, that the virial coefficients and, therefore, the equation of state and excess free energies for the fluid phase be evaluated analytically. A recent study <ref name="JCP">[http://dx.doi.org/10.1063/1.4967775 R. Paula Leite, R. Freitas, R. Azevedo and M. de Koning "The Uhlenbeck-Ford model: Exact virial coefficients and application as a reference system in fluid-phase free-energy calculations", Journal of Chemical Physics '''145''', 194101 (2016).]</ref> showed that this model can be used as a reference system for fluid-phase free-energy calculations. | ||
| Line 14: | Line 14: | ||
* <math> \sigma </math> is a length-scale parameter. | * <math> \sigma </math> is a length-scale parameter. | ||
Note that increasing the value of p gives rise to a stronger repulsion. | Note that increasing the value of <math>p</math> gives rise to a stronger repulsion. | ||
== Equation of state == | == Equation of state == | ||
The equation of state for the UFM fluid using virial expansion has recently been studied by Paula Leite, Freitas, Azevedo and de Koning <ref name="JCP"></ref> and is given by | The [[Equations of state |equation of state]] for the UFM fluid using [[Virial equation of state | virial expansion]] has recently been studied by Paula Leite, Freitas, Azevedo and de Koning <ref name="JCP"></ref> and is given by | ||
:<math>\beta bP_{\rm UF}(x,p) = x + \sum_{n=2}^{\infty} \tilde{B}_n(p) \,x^n</math>, | :<math>\beta bP_{\rm UF}(x,p) = x + \sum_{n=2}^{\infty} \tilde{B}_n(p) \,x^n</math>, | ||
where | where | ||
*<math>P_{\rm UF}(x,p)</math> is the system pressure. | *<math>P_{\rm UF}(x,p)</math> is the system [[pressure]]. | ||
*<math>b \equiv \frac{1}{2}(\pi\sigma^2)^{3/2} </math> is a constant. | *<math>b \equiv \frac{1}{2}(\pi\sigma^2)^{3/2} </math> is a constant. | ||
*<math>x \equiv b\rho </math> is an adimensional variable. | *<math>x \equiv b\rho </math> is an adimensional variable. | ||
| Line 32: | Line 32: | ||
== Excess Helmholtz free-energy == | == Excess Helmholtz free-energy == | ||
The excess Helmholtz free-energy expression for the UFM, which can be obtained integrating the equation of state with respect to volume <ref name="JCP"></ref>, is given by | The excess [[Helmholtz energy function | Helmholtz free-energy]] expression for the UFM, which can be obtained integrating the equation of state with respect to volume <ref name="JCP"></ref>, is given by | ||
:<math>\frac{\beta F^{\rm (exc)}_{\rm UF}(x,p)}{N} =\sum_{n=1}^{\infty} \frac{\tilde{B}_{n+1}(p)}{n} \,x^n</math>, | :<math>\frac{\beta F^{\rm (exc)}_{\rm UF}(x,p)}{N} =\sum_{n=1}^{\infty} \frac{\tilde{B}_{n+1}(p)}{n} \,x^n</math>, | ||
| Line 41: | Line 41: | ||
== Virial coefficients == | == Virial coefficients == | ||
As the virial coefficients for the UFM can be calculated analytically, in principle, Paula Leite, Freitas, Azevedo and de Koning <ref name="JCP"></ref> recalculated the first eight coefficients previously known <math>(\tilde{B}_2</math> to <math>\tilde{B}_9)</math> and obtained values for four new coefficients <math>(\tilde{B}_{10}</math> to <math>\tilde{B}_{13})</math> for <math>p=1</math>. Some coefficients for <math>p=2</math> and <math>p=10</math> are also shown in the table below. | As the [[Virial equation of state | virial coefficients]] for the UFM can be calculated analytically, in principle, Paula Leite, Freitas, Azevedo and de Koning <ref name="JCP"></ref> recalculated the first eight coefficients previously known <math>(\tilde{B}_2</math> to <math>\tilde{B}_9)</math> and obtained values for four new coefficients <math>(\tilde{B}_{10}</math> to <math>\tilde{B}_{13})</math> for <math>p=1</math>. Some coefficients for <math>p=2</math> and <math>p=10</math> are also shown in the table below. | ||
Table 1: Numerical representation of the exact virial coefficients for the UFM. | Table 1: Numerical representation of the exact virial coefficients for the UFM. | ||
| Line 74: | Line 74: | ||
== Numerical representations == | == Numerical representations == | ||
Due to the number of integrals needed to compute the virial coefficients, a numerical representation using molecular dynamics data is usually adopted. In this sense, extensive molecular dynamics simulations have been performed and using a piece-wise fitting procedure, called cubic splines, numerical expressions for the UFM's equation of state were constructed. Moreover, this representation also leads to an excellent description for the excess Helmholtz free energy <math>F^{\rm (exc)}_{\rm UF}(x,p)</math>. | Due to the number of integrals needed to compute the virial coefficients, a numerical representation using molecular dynamics data is usually adopted. In this sense, extensive [[molecular dynamics]] simulations have been performed and using a piece-wise fitting procedure, called cubic splines, numerical expressions for the UFM's equation of state were constructed. Moreover, this representation also leads to an excellent description for the excess Helmholtz free energy <math>F^{\rm (exc)}_{\rm UF}(x,p)</math>. | ||
All spline representations of equations of state and excess Helmholtz free energies are available by means of the | All spline representations of equations of state and excess Helmholtz free energies are available by means of the python script ''ufGenerator.py'' supplied in the supplementary material of Paula Leite ''et al''. paper <ref name="JCP"></ref>. | ||
== Phase diagram == | == Phase diagram == | ||
The phase diagram of the UFM in the <math>(p^{-1},P_{\rm UF}^*)</math> has been constructed by Paula Leite, Santos-Flórez and de Koning <ref name="PRE">[https://doi.org/10.1103/PhysRevE.96.032115 R. Paula Leite, P. A. Santos-Flórez and M. de Koning "Uhlenbeck-Ford model: Phase diagram and corresponding-states analysis", Physical Review E '''96''', 032115 (2017).]</ref>. Beyond the fluid phase, body-centered-cubic (bcc) and face-centered-cubic (fcc) are the only thermodynamically stable crystalline phases. These three phases meet each other at a triple point. Furthermore, they reported the existence of two reentrant transition sequences as a function of the number density, one featuring a fluid-bcc-fluid succession and another displaying a bcc-fcc-bcc sequence near the triple point. | The [[Phase diagrams | phase diagram]] of the UFM in the <math>(p^{-1},P_{\rm UF}^*)</math> has been constructed by Paula Leite, Santos-Flórez and de Koning <ref name="PRE">[https://doi.org/10.1103/PhysRevE.96.032115 R. Paula Leite, P. A. Santos-Flórez and M. de Koning "Uhlenbeck-Ford model: Phase diagram and corresponding-states analysis", Physical Review E '''96''', 032115 (2017).]</ref>. Beyond the fluid phase, [[Building up a body centered cubic lattice |body-centered-cubic]] (bcc) and [[Building up a face centered cubic lattice | face-centered-cubic]] (fcc) are the only thermodynamically stable crystalline phases. These three phases meet each other at a [[triple point]]. Furthermore, they reported the existence of two reentrant transition sequences as a function of the number density, one featuring a fluid-bcc-fluid succession and another displaying a bcc-fcc-bcc sequence near the triple point. | ||
== Reference system == | == Reference system == | ||
The UFM can be used as a reference system for fluid-phase free-energy calculations. Examples of these kind of calculations using thermodynamic integration | The UFM can be used as a reference system for fluid-phase free-energy calculations. Examples of these kind of calculations using [[thermodynamic integration]] techniques for different natures of fluid description ([[Lennard-Jones model |Lennard-Jones]] and [[Stillinger-Weber potential | Stillinger-Weber]]) can be found in Paula Leite ''et al''. paper <ref name="JCP"></ref>. The typical values for <math>p</math> are <math>50 \sim 100.</math>. | ||
==References== | ==References== | ||
Revision as of 11:39, 17 October 2017
The Uhlenbeck-Ford model (UFM), originally named Gaussian gas [1], was proposed by G. Uhlenbeck and G. Ford [2] for the theoretical study of imperfect gases. This model is characterized by an ultrasoft, purely repulsive pairwise interaction potential that diverges logarithmically at the origin and features an energy scale that coincides with the thermal energy unit where is the Boltzmann constant and the absolute temperature. The particular functional form of the potential permits, in principle, that the virial coefficients and, therefore, the equation of state and excess free energies for the fluid phase be evaluated analytically. A recent study [3] showed that this model can be used as a reference system for fluid-phase free-energy calculations.
Functional form
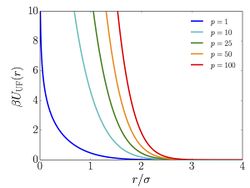
The pairwise interaction potential associated with the Uhlenbeck-Ford model is given by
- ,
where
- is a scaling factor.
- is the well depth (energy).
- is the inter particle distance.
- is a length-scale parameter.
Note that increasing the value of gives rise to a stronger repulsion.
Equation of state
The equation of state for the UFM fluid using virial expansion has recently been studied by Paula Leite, Freitas, Azevedo and de Koning [3] and is given by
- ,
where
- is the system pressure.
- is a constant.
- is an adimensional variable.
- are the reduced virial coefficients.
Note that, due to the functional form of the potential, the equation of state for the UFM fluid can be specified in terms of a function of a single adimensional variable , regardless of the length-scale and absolute temperature , i.é., the virial coefficients are independent of the absolute temperature.
Excess Helmholtz free-energy
The excess Helmholtz free-energy expression for the UFM, which can be obtained integrating the equation of state with respect to volume [3], is given by
- ,
where
- is the excess Helmholtz free energy.
- is the number of particles.
Virial coefficients
As the virial coefficients for the UFM can be calculated analytically, in principle, Paula Leite, Freitas, Azevedo and de Koning [3] recalculated the first eight coefficients previously known to and obtained values for four new coefficients to Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_{13})} for Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p=1} . Some coefficients for Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p=2} and Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p=10} are also shown in the table below.
Table 1: Numerical representation of the exact virial coefficients for the UFM.
p=1 p=2 p=10 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_2} 1 1.646 446 609 40 4.014 383 975 40 0.256 600 119 639 833 673 11 0.943 195 827 15 8.031 625 170 36 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_4} -0.125 459 957 055 044 678 34 -0.334 985 180 88 5.791 113 868 79 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_5} 0.013 325 655 173 205 441 00 -0.303 688 574 00 -7.658 371 037 27 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_6} 0.038 460 935 830 869 671 55 0.395 857 781 68 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_7} -0.033 083 442 903 149 717 39 0.053 655 130 98 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_8} 0.004 182 418 769 682 387 35 -0.408 481 406 00 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_9} 0.015 197 607 195 500 874 66 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_{10}} -0.013 849 654 134 575 142 93 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_{11}} 0.001 334 757 917 110 966 11 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_{12}} 0.007 604 327 248 812 125 87 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \tilde{B}_{13}} -0.006 726 441 408 781 588 25
Numerical representations
Due to the number of integrals needed to compute the virial coefficients, a numerical representation using molecular dynamics data is usually adopted. In this sense, extensive molecular dynamics simulations have been performed and using a piece-wise fitting procedure, called cubic splines, numerical expressions for the UFM's equation of state were constructed. Moreover, this representation also leads to an excellent description for the excess Helmholtz free energy Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle F^{\rm (exc)}_{\rm UF}(x,p)} .
All spline representations of equations of state and excess Helmholtz free energies are available by means of the python script ufGenerator.py supplied in the supplementary material of Paula Leite et al. paper [3].
Phase diagram
The phase diagram of the UFM in the Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle (p^{-1},P_{\rm UF}^*)} has been constructed by Paula Leite, Santos-Flórez and de Koning [4]. Beyond the fluid phase, body-centered-cubic (bcc) and face-centered-cubic (fcc) are the only thermodynamically stable crystalline phases. These three phases meet each other at a triple point. Furthermore, they reported the existence of two reentrant transition sequences as a function of the number density, one featuring a fluid-bcc-fluid succession and another displaying a bcc-fcc-bcc sequence near the triple point.
Reference system
The UFM can be used as a reference system for fluid-phase free-energy calculations. Examples of these kind of calculations using thermodynamic integration techniques for different natures of fluid description (Lennard-Jones and Stillinger-Weber) can be found in Paula Leite et al. paper [3]. The typical values for Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p} are Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 50 \sim 100.} .
References
- ↑ D. McQuarrie, Statistical Mechanics (University Science Books, 2000).
- ↑ G. Uhlenbeck and G. Ford, in Studies in Statistical Mechanics— The Theory of Linear Graphs with Application to the Theory of the Virial Development of the Properties of Gases, edited by G. E. Uhlenbeck and J. de Boer (North-Holland, Amsterdam, 1962), Vol. 2.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 R. Paula Leite, R. Freitas, R. Azevedo and M. de Koning "The Uhlenbeck-Ford model: Exact virial coefficients and application as a reference system in fluid-phase free-energy calculations", Journal of Chemical Physics 145, 194101 (2016).
- ↑ 4.0 4.1 R. Paula Leite, P. A. Santos-Flórez and M. de Koning "Uhlenbeck-Ford model: Phase diagram and corresponding-states analysis", Physical Review E 96, 032115 (2017).





















