SPC/Fw model of water: Difference between revisions
Carl McBride (talk | contribs) m (Added a recent publication) |
(Minor edit of units of Ka,b constants) |
||
| Line 1: | Line 1: | ||
The '''SPC/Fw''' is a flexible variant of the rigid [[SPC]] model for [[water]] | The '''SPC/Fw''' is a flexible variant of the rigid [[SPC]] model for [[water]] | ||
UNIQ2c14b0eded24b1ba-ref-00000023-QINU. | |||
This model has also been re-parametrised for quantum simulations, adopting the name '''q-SPC/Fw''' | This model has also been re-parametrised for quantum simulations, adopting the name '''q-SPC/Fw''' | ||
UNIQ2c14b0eded24b1ba-ref-00000024-QINU. | |||
The model is given by the intra-molecular component (Eq. 2 of | The model is given by the intra-molecular component (Eq. 2 of UNIQ2c14b0eded24b1ba-ref-00000025-QINU): | ||
: | :UNIQ2c14b0eded24b1ba-math-00000026-QINU | ||
and the inter-molecular component (Eq. 3 of | and the inter-molecular component (Eq. 3 of UNIQ2c14b0eded24b1ba-ref-00000027-QINU): | ||
: | :UNIQ2c14b0eded24b1ba-math-00000028-QINU | ||
The parameters for both of these models are given in the following table (Table I of | The parameters for both of these models are given in the following table (Table I of UNIQ2c14b0eded24b1ba-ref-00000029-QINU): | ||
[[Image:Thee_site_water_model.png|center|400px]] | [[Image:Thee_site_water_model.png|center|400px]] | ||
| Line 18: | Line 18: | ||
{| style="width:75%; height:100px" border="1" | {| style="width:75%; height:100px" border="1" | ||
|- | |- | ||
| Model || | | Model || UNIQ2c14b0eded24b1ba-math-0000002A-QINU || UNIQ2c14b0eded24b1ba-math-0000002B-QINU (Å)|| UNIQ2c14b0eded24b1ba-math-0000002C-QINU || UNIQ2c14b0eded24b1ba-math-0000002D-QINU (deg)|| UNIQ2c14b0eded24b1ba-math-0000002E-QINU (Å)|| UNIQ2c14b0eded24b1ba-math-0000002F-QINU (kcal mol<sup>-1</sup>)|| q(O) (e) || q(H) (e) | ||
|- | |- | ||
| SPC/Fw || 1059.162 || 1.012 || 75.90 || 113.24 || 3.165492 || 0.1554253 || -0.82 || 0.41 | | SPC/Fw || 1059.162 || 1.012 || 75.90 || 113.24 || 3.165492 || 0.1554253 || -0.82 || 0.41 | ||
| Line 24: | Line 24: | ||
| q-SPC/Fw || 1059.162 || 1.000 || 75.90 || 112.0 || 3.165492 || 0.1554252 || -0.84 || 0.42 | | q-SPC/Fw || 1059.162 || 1.000 || 75.90 || 112.0 || 3.165492 || 0.1554252 || -0.84 || 0.42 | ||
|} | |} | ||
where the units of < | where the units of UNIQ2c14b0eded24b1ba-math-00000030-QINU and UNIQ2c14b0eded24b1ba-math-00000031-QINU are kcal.mol<sup>-1</sup>Å<sup>-2</sup> and kcal.mol<sup>-1</sup>rad.<sup>-2</sup> respectively. | ||
==Dielectric constant== | ==Dielectric constant== | ||
The dielectric constant has been calculated by Raabe and Sadus | The dielectric constant has been calculated by Raabe and Sadus UNIQ2c14b0eded24b1ba-ref-00000032-QINU. | ||
==References== | ==References== | ||
UNIQ2c14b0eded24b1ba-references-00000033-QINU | |||
{{numeric}} | {{numeric}} | ||
[[category: water]] | [[category: water]] | ||
[[category: models]] | [[category: models]] | ||
Revision as of 00:04, 28 December 2014
The SPC/Fw is a flexible variant of the rigid SPC model for water ?UNIQ2c14b0eded24b1ba-ref-00000023-QINU?. This model has also been re-parametrised for quantum simulations, adopting the name q-SPC/Fw ?UNIQ2c14b0eded24b1ba-ref-00000024-QINU?. The model is given by the intra-molecular component (Eq. 2 of ?UNIQ2c14b0eded24b1ba-ref-00000025-QINU?):
- ?UNIQ2c14b0eded24b1ba-math-00000026-QINU?
and the inter-molecular component (Eq. 3 of ?UNIQ2c14b0eded24b1ba-ref-00000027-QINU?):
- ?UNIQ2c14b0eded24b1ba-math-00000028-QINU?
The parameters for both of these models are given in the following table (Table I of ?UNIQ2c14b0eded24b1ba-ref-00000029-QINU?):
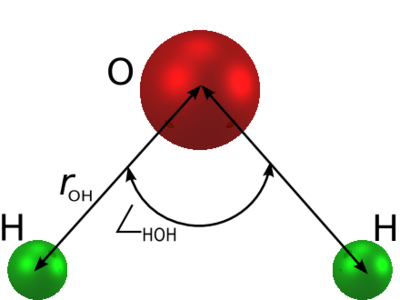
| Model | ?UNIQ2c14b0eded24b1ba-math-0000002A-QINU? | ?UNIQ2c14b0eded24b1ba-math-0000002B-QINU? (Å) | ?UNIQ2c14b0eded24b1ba-math-0000002C-QINU? | ?UNIQ2c14b0eded24b1ba-math-0000002D-QINU? (deg) | ?UNIQ2c14b0eded24b1ba-math-0000002E-QINU? (Å) | ?UNIQ2c14b0eded24b1ba-math-0000002F-QINU? (kcal mol-1) | q(O) (e) | q(H) (e) |
| SPC/Fw | 1059.162 | 1.012 | 75.90 | 113.24 | 3.165492 | 0.1554253 | -0.82 | 0.41 |
| q-SPC/Fw | 1059.162 | 1.000 | 75.90 | 112.0 | 3.165492 | 0.1554252 | -0.84 | 0.42 |
where the units of ?UNIQ2c14b0eded24b1ba-math-00000030-QINU? and ?UNIQ2c14b0eded24b1ba-math-00000031-QINU? are kcal.mol-1Å-2 and kcal.mol-1rad.-2 respectively.
Dielectric constant
The dielectric constant has been calculated by Raabe and Sadus ?UNIQ2c14b0eded24b1ba-ref-00000032-QINU?.
References
?UNIQ2c14b0eded24b1ba-references-00000033-QINU?