SCPDP model of water: Difference between revisions
Jump to navigation
Jump to search
m (Added a little more material.) |
mNo edit summary |
||
| Line 2: | Line 2: | ||
The '''SCPDP''' model | The '''SCPDP''' model | ||
<ref>[http://dx.doi.org/10.1063/1.472718 Ariel A. Chialvo and Peter T. Cummings "Engineering a simple polarizable model for the molecular simulation of water applicable over wide ranges of state conditions", Journal of Chemical Physics '''105''' pp. 8274-8281 (1996)]</ref> | <ref>[http://dx.doi.org/10.1063/1.472718 Ariel A. Chialvo and Peter T. Cummings "Engineering a simple polarizable model for the molecular simulation of water applicable over wide ranges of state conditions", Journal of Chemical Physics '''105''' pp. 8274-8281 (1996)]</ref> | ||
is | is similar to the [[SPC]] model for [[water]]. It is polarisable, and has a permanent [[dipole moment]] of 1.85D. | ||
==Parameters== | ==Parameters== | ||
[[Image:Thee_site_water_model.png|center|400px]] | [[Image:Thee_site_water_model.png|center|400px]] | ||
Revision as of 19:24, 10 June 2009
The SCPDP model [1] is similar to the SPC model for water. It is polarisable, and has a permanent dipole moment of 1.85D.
Parameters
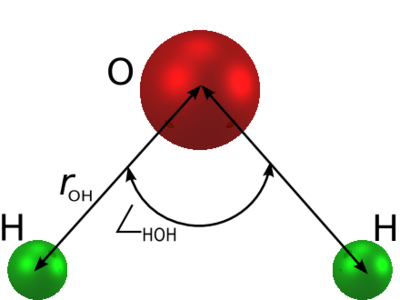
| parameter | value |
| kJ mol-1 | |
| (charge neutrality) |
Vapour–liquid equilibrium
- Milan Predota , Ariel A. Chialvoa, and Peter T. Cummings "On the determination of the vapor–liquid envelope for polarizable models by Monte Carlo simulation", Fluid Phase Equilibria 183-184 pp. 295-300 (2001)
- J. L. Rivera, M. Predota, A. A. Chialvo, and P. T. Cummings "Vapor–liquid equilibrium simulations of the SCPDP model of water", Chemical Physics Letters 357 pp. 189-194 (2002)











