Anisotropic particles with tetrahedral symmetry: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| Line 6: | Line 6: | ||
diameter). Interestingly, and differently from the isotropic case, the supersaturation of the fluid at the critical point does not significantly increase upon going toward the adhesive (vanishing interaction range) limit. | diameter). Interestingly, and differently from the isotropic case, the supersaturation of the fluid at the critical point does not significantly increase upon going toward the adhesive (vanishing interaction range) limit. | ||
[[Image:romanojpcb09.gif]] | |||
Revision as of 18:07, 28 November 2009
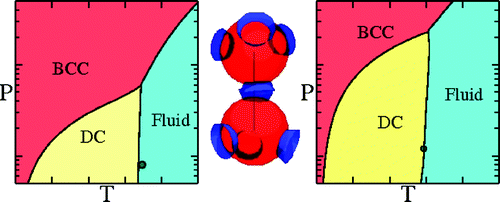
The phase diagram of tetrahedral, patchy particles [1] exhibits the following solid phases: Diamond Crystal, Body Centered Cubic and Face Centered Cubic. The gas-liquid critical point becomes metastable with respect to the Diamond Crystal when the range of the interaction becomes short (roughly less than 15% of the diameter). Interestingly, and differently from the isotropic case, the supersaturation of the fluid at the critical point does not significantly increase upon going toward the adhesive (vanishing interaction range) limit.
References
- ↑ [http://dx.doi.org/10.1021/jp9081905 F. Romano, E. Sanz and F. Sciortino "Role of the Range in the Fluid−Crystal Coexistence for a Patchy Particle Model", J. Phys. Chem. B 113 pp. 15133–15136 (2009)]