TIP4P/FQ model of water: Difference between revisions
Jump to navigation
Jump to search
m (Started a parameters section.) |
Carl McBride (talk | contribs) m (Added melting point) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 13: | Line 13: | ||
| --- || --- || --- || --- || --- || --- || --- || --- | | --- || --- || --- || --- || --- || --- || --- || --- | ||
|} | |} | ||
==Melting point== | |||
The [[Ice Ih]]-[[water]] melting point has been calculated to be <math>303\pm 8~K</math> <ref>[http://dx.doi.org/10.1016/j.jcrysgro.2006.04.077 Benjamin F. Nicholson, Paulette Clancy and Steven W. Rick "The interface response function and melting point of the prism interface of ice Ih using a fluctuating charge model (TIP4P-FQ)", Journal of Crystal Growth '''293''' pp. 78-85 (2006)] </ref>. | |||
==References== | ==References== | ||
<references/> | <references/> | ||
[[category: water]] | [[category: water]] | ||
[[category: models]] | [[category: models]] | ||
Latest revision as of 16:29, 15 June 2011
The TIP4P/FQ model [1] is a variant of the TIP4P model, which now incorporates fluctuating charges.
Parameters[edit]
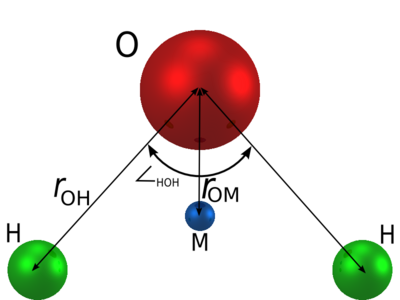
| (Å) | HOH , deg | (Å) | (K) | q(O) (e) | q(H) (e) | q(M) (e) | (Å) |
| --- | --- | --- | --- | --- | --- | --- | --- |
Melting point[edit]
The Ice Ih-water melting point has been calculated to be [2].
References[edit]
- ↑ Steven W. Rick, Steven J. Stuart, and B. J. Berne "Dynamical fluctuating charge force fields: Application to liquid water", Journal of Chemical Physics 101 pp. 6141-6156 (1994)
- ↑ Benjamin F. Nicholson, Paulette Clancy and Steven W. Rick "The interface response function and melting point of the prism interface of ice Ih using a fluctuating charge model (TIP4P-FQ)", Journal of Crystal Growth 293 pp. 78-85 (2006)





