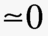
|
This article is a 'stub' page, it has no, or next to no, content. It is here at the moment to help form part of the structure of SklogWiki. If you add sufficient material to this article then please remove the {{Stub-general}} template from this page.
|
The Debye length for a general electrolyte is given by [1]

where  is the ionic strength of the electrolyte.
is the ionic strength of the electrolyte.
See also[edit]
References[edit]

