SPC model of water: Difference between revisions
Jump to navigation
Jump to search
m (Slight tidy.) |
m (Changed internal link.) |
||
| Line 1: | Line 1: | ||
The '''simple point charge''' (SPC) model | The '''simple point charge''' (SPC) model | ||
<ref>H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, in: Intermolecular Forces (B. Pullman, ed.), Reidel, Dordrecht, p. 331 (1981)</ref> | <ref>H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, in: Intermolecular Forces (B. Pullman, ed.), Reidel, Dordrecht, p. 331 (1981)</ref> | ||
is an [[ | is an [[water models | empirical model of water]]. The molecule is modelled as | ||
a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range [[Lennard-Jones model | Lennard-Jones]] sites, situated on the oxygen atoms. The parameters are as follows: | a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range [[Lennard-Jones model | Lennard-Jones]] sites, situated on the oxygen atoms. The parameters are as follows: | ||
[[Image:Thee_site_water_model.png|center|400px]] | [[Image:Thee_site_water_model.png|center|400px]] | ||
Revision as of 12:02, 12 June 2009
The simple point charge (SPC) model [1] is an empirical model of water. The molecule is modelled as a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range Lennard-Jones sites, situated on the oxygen atoms. The parameters are as follows:
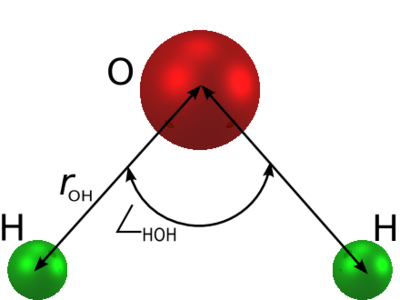
| parameter | value |
| Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \sigma} | Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 3.166 {\mathrm {\AA}}} |
| Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \epsilon} | Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 0.650} kJ mol-1 |
| Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 1.000\mathrm{\AA}} | |
| Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \angle_\mathrm{HOH}} | Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 109.47^{\circ}} |
| Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle q_{\mathrm{O}}} | |
| Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle q_{\mathrm{H}}} | Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle q_{\mathrm{O}}/2} (charge neutrality) |
The SPC model has a dipole moment of 2.27 D.
Related models
Over the years a number of variants of the SPC model have been published:
References
- ↑ H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, in: Intermolecular Forces (B. Pullman, ed.), Reidel, Dordrecht, p. 331 (1981)

