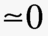
|
This article is a 'stub' page, it has no, or next to no, content. It is here at the moment to help form part of the structure of SklogWiki. If you add sufficient material to this article then please remove the {{Stub-general}} template from this page.
|
The temperature of a system in classical thermodynamics is intimately related to the zeroth law of thermodynamics; two systems having to have the same temperature if they are to be in thermal equilibrium (i.e. there is no net heat flow between them).
However, it is most useful to have a temperature scale.
By making use of the ideal gas law one can define an absolute temperature

having the SI units of Kelvin (named in honour of William Thomson).

Kinetic temperature
Configurational temperature
Non-equilibrium temperature
References

