SPC model of water: Difference between revisions
Jump to navigation
Jump to search
(dipole moment added) |
Carl McBride (talk | contribs) m (Updated internal link) |
||
| Line 1: | Line 1: | ||
{{Stub-water}} | {{Stub-water}} | ||
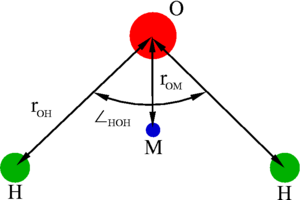
The '''simple point charge''' (SPC) model is an [[ | The '''simple point charge''' (SPC) model is an [[List of empirical water models | empirical model of water]]. The molecule is modelled as | ||
a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range [[Lennard-Jones model | Lennard-Jones]] sites, situated on the oxygen atoms. The parameters are as follows: | a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range [[Lennard-Jones model | Lennard-Jones]] sites, situated on the oxygen atoms. The parameters are as follows: | ||
[[Image:Water_empirical1.png|center|300px]] | [[Image:Water_empirical1.png|center|300px]] | ||
Revision as of 13:23, 5 September 2008
The simple point charge (SPC) model is an empirical model of water. The molecule is modelled as a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range Lennard-Jones sites, situated on the oxygen atoms. The parameters are as follows:
| parameter | value |
| kJ mol-1 | |
| (charge neutrality) | |
| (charge sits on oxygen) |
The SPC model has a dipole moment of 2.27 D.
References
- H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, in: Intermolecular Forces (B. Pullman, ed.), Reidel, Dordrecht, p. 331 (1981).













