SPC model of water: Difference between revisions
Jump to navigation
Jump to search
mNo edit summary |
(dipole moment added) |
||
| Line 22: | Line 22: | ||
|} | |} | ||
The SPC model has a [[dipole moment]] of 2.27 D. | |||
Revision as of 12:31, 26 May 2008
The simple point charge (SPC) model is an empirical model of water. The molecule is modelled as a rigid isosceles triangle, having charges situated on each of the three atoms. Apart from Coulombic interactions, the molecules interact via long-range Lennard-Jones sites, situated on the oxygen atoms. The parameters are as follows:
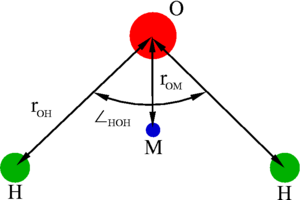
| parameter | value |
| kJ mol-1 | |
| (charge neutrality) | |
| (charge sits on oxygen) |
The SPC model has a dipole moment of 2.27 D.
References
- H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren and J. Hermans, in: Intermolecular Forces (B. Pullman, ed.), Reidel, Dordrecht, p. 331 (1981).













