Phase diagrams of water
| This SklogWiki entry needs to be rewritten at some point to improve coherence and readability. |
Experimental
The following are references for the equation of state of water.
References
- The phase diagram of water to 45,000 kg/cm2. P. W. Bridgman, Journal of Chemical Physics 5: 964-966 (1937)
- A. Saul and W. Wagner "International Equations for the Saturation Properties of Ordinary Water Substance", Journal of Physical and Chemical Reference Data 16 pp. 893-901 (1987)
- H. Sato, M. Uematsu, K. Watanabe, A. Saul and W. Wagner "New International Skeleton Tables for the Thermodynamic Properties of Ordinary Water Substance", Journal of Physical and Chemical Reference Data 17 pp. 1439-1540 (1988)
- A. Saul and W. Wagner "A Fundamental Equation for Water Covering the Range from the Melting Line to 1273 K at Pressures up to 25 000 MPa", Journal of Physical and Chemical Reference Data 18 pp. 1537-1564 (1989)
- H. Sato, K. Watanabe, J. M. H. Levelt Sengers, J. S. Gallagher, P. G. Hill, J. Straub and W. Wagner "Sixteen Thousand Evaluated Experimental Thermodynamic Property Data for Water and Steam", Journal of Physical and Chemical Reference Data 20 pp. 1023-1044 (1991)
- Wolfgang Wagner and A. Pruss "International Equations for the Saturation Properties of Ordinary Water Substance. Revised According to the International Temperature Scale of 1990. Addendum to J. Phys. Chem. Ref. Data 16, 893 (1987)", Journal of Physical and Chemical Reference Data 22 pp. 783-787 (1993)
- Wolfgang Wagner, A. Saul, and A. Pruss "International Equations for the Pressure Along the Melting and Along the Sublimation Curve of Ordinary Water Substance", Journal of Physical and Chemical Reference Data 23 pp. 515-527 (1994)
- W. Wagner and A. Pruß "The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use", Journal of Physical and Chemical Reference Data 31 pp. 387-535 (2002)
- Interactive 3D phase diagrams using Jmol. A. Herráez, R. M. Hanson, and L. Glasser, Journal of Chemical Education 86: 566 (2009) and website
- Water, water, everywhere: Phase diagrams of ordinary water substance. L. Glasser, Journal of Chemical Education 81: 414-418 (2004)
Theoretical
TIP4P/2005 model
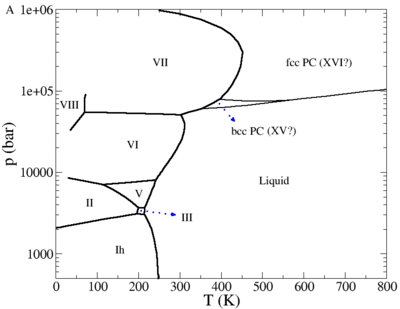
The phase diagram of the TIP4P/2005 model is given in a publication by Abascal, Sanz and Vega.
and for negative pressures in the publication
Liquid-vapour equilibria
Plastic crystal phases
Recent simulations have suggested the possibility of a plastic crystal phase or phases for water.
- J. L. Aragones, M. M. Conde, E. G. Noya and C. Vega "The phase diagram of water at high pressures as obtained by computer simulations of the TIP4P/2005 model: the appearance of a plastic crystal phase", Physical Chemistry Chemical Physics 11 pp. 543- (2009)
- J. L. Aragones and C. Vega "Plastic crystal phases of simple water models", Journal of Chemical Physics 130 244504 (2009)
References
- Phase diagram of water from computer simulation. E. Sanz, C. Vega, J. L. F. Abascal, and L. G. MacDowell, Physical Review Letters 92: 255701 (2004)