Phase diagrams of water: Difference between revisions
Jump to navigation
Jump to search
Carl McBride (talk | contribs) m (Slight tidy. (Note: many DOI are not yet active)) |
Carl McBride (talk | contribs) m (Changed some title levels.) |
||
| Line 28: | Line 28: | ||
*[http://dx.doi.org/10.1063/1.3156856 J. L. Aragones and C. Vega "Plastic crystal phases of simple water models", Journal of Chemical Physics '''130''' 244504 (2009)] | *[http://dx.doi.org/10.1063/1.3156856 J. L. Aragones and C. Vega "Plastic crystal phases of simple water models", Journal of Chemical Physics '''130''' 244504 (2009)] | ||
=References= | |||
<references/> | <references/> | ||
=External links= | |||
*[http://www.iapws.org/ The International Association for the Properties of Water and Steam] | *[http://www.iapws.org/ The International Association for the Properties of Water and Steam] | ||
[[category:phase diagrams]] | [[category:phase diagrams]] | ||
[[category:water]] | [[category:water]] | ||
[[category: equations of state]] | [[category: equations of state]] | ||
Revision as of 13:55, 5 November 2009
Experimental
The following are references for the experimental equation of state of water.
References
- P. W. Bridgman "The phase diagram of water to 45,000 kg/cm2", Journal of Chemical Physics 5 pp. 964-966 (1937)
- A. Saul and W. Wagner "International Equations for the Saturation Properties of Ordinary Water Substance", Journal of Physical and Chemical Reference Data 16 pp. 893-901 (1987)
- H. Sato, M. Uematsu, K. Watanabe, A. Saul and W. Wagner "New International Skeleton Tables for the Thermodynamic Properties of Ordinary Water Substance", Journal of Physical and Chemical Reference Data 17 pp. 1439-1540 (1988)
- A. Saul and W. Wagner "A Fundamental Equation for Water Covering the Range from the Melting Line to 1273 K at Pressures up to 25 000 MPa", Journal of Physical and Chemical Reference Data 18 pp. 1537-1564 (1989)
- H. Sato, K. Watanabe, J. M. H. Levelt Sengers, J. S. Gallagher, P. G. Hill, J. Straub and W. Wagner "Sixteen Thousand Evaluated Experimental Thermodynamic Property Data for Water and Steam", Journal of Physical and Chemical Reference Data 20 pp. 1023-1044 (1991)
- Wolfgang Wagner and A. Pruss "International Equations for the Saturation Properties of Ordinary Water Substance. Revised According to the International Temperature Scale of 1990. Addendum to J. Phys. Chem. Ref. Data 16, 893 (1987)", Journal of Physical and Chemical Reference Data 22 pp. 783-787 (1993)
- Wolfgang Wagner, A. Saul, and A. Pruss "International Equations for the Pressure Along the Melting and Along the Sublimation Curve of Ordinary Water Substance", Journal of Physical and Chemical Reference Data 23 pp. 515-527 (1994)
- W. Wagner and A. Pruß "The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use", Journal of Physical and Chemical Reference Data 31 pp. 387-535 (2002)
- A. Herráez, R. M. Hanson, and L. Glasser "Interactive 3D phase diagrams using Jmol", Journal of Chemical Education 86 pp. 566- (2009) and website
- L. Glasser "Water, water, everywhere: Phase diagrams of ordinary water substance", Journal of Chemical Education 81 pp. 414-418 (2004)
Theoretical
Computer simulation techniques have now reached the stage where it is possible to calculate the entire phase diagram for a particular model of water. For example, see Ref [1]
TIP4P/2005 model
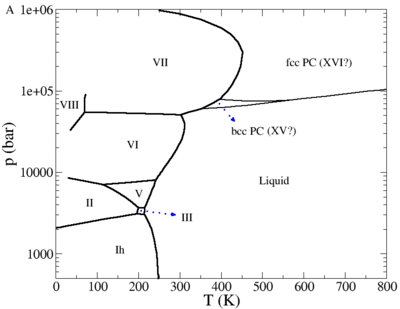
The phase diagram of the TIP4P/2005 model is given in a publication by Abascal, Sanz and Vega.
and for negative pressures in the publication
Liquid-vapour equilibria
Plastic crystal phases
Recent simulations have suggested the possibility of a plastic crystal phase or phases for water.
- J. L. Aragones, M. M. Conde, E. G. Noya and C. Vega "The phase diagram of water at high pressures as obtained by computer simulations of the TIP4P/2005 model: the appearance of a plastic crystal phase", Physical Chemistry Chemical Physics 11 pp. 543- (2009)
- J. L. Aragones and C. Vega "Plastic crystal phases of simple water models", Journal of Chemical Physics 130 244504 (2009)