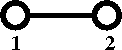Mayer f-function: Difference between revisions
Jump to navigation
Jump to search
Carl McBride (talk | contribs) No edit summary |
Carl McBride (talk | contribs) m (Defined as) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
The '''Mayer ''f''-function''', or ''f-bond'' is defined as (Ref. 1 Chapter 13 Eq. 13.2): | |||
:<math>f_{ | :<math>f_{12}=f({\mathbf r}_{12}) := \exp\left(-\frac{\Phi_{12}(r)}{k_BT}\right) -1 </math> | ||
where | where | ||
* <math>k_B</math> is the [[Boltzmann constant]] | * <math>k_B</math> is the [[Boltzmann constant]]. | ||
* <math>T</math> is the temperature | * <math>T</math> is the [[temperature]]. | ||
* <math> | * <math>\Phi_{12}(r)</math> is the [[intermolecular pair potential]]. | ||
In other words, the Mayer function is the [[Boltzmann factor]] of the interaction potential, | |||
minus one. | |||
[[Cluster diagrams | Diagrammatically]] the Mayer ''f''-function is written as | |||
:[[Image:Mayer_f_function.png]] | |||
==Hard sphere model== | |||
For the [[hard sphere model]] the Mayer ''f''-function becomes: | |||
: <math> | |||
f_{12}= \left\{ \begin{array}{lll} | |||
-1 & ; & r_{12} \leq \sigma ~~({\rm overlap})\\ | |||
0 & ; & r_{12} > \sigma ~~({\rm no~overlap})\end{array} \right. | |||
</math> | |||
where <math>\sigma</math> is the hard sphere diameter. | |||
==References== | ==References== | ||
# Joseph Edward Mayer and Maria Goeppert Mayer "Statistical Mechanics" John Wiley and Sons (1940) | |||
#[http://dx.doi.org/10.1063/1.1723631 Joseph E. Mayer "Contribution to Statistical Mechanics", Journal of Chemical Physics '''10''' pp. 629-643 (1942)] | |||
[[Category: Statistical mechanics]] | [[Category: Statistical mechanics]] | ||
[[Category: Integral equations]] | [[Category: Integral equations]] | ||
Latest revision as of 19:50, 20 February 2015
The Mayer f-function, or f-bond is defined as (Ref. 1 Chapter 13 Eq. 13.2):
where
- is the Boltzmann constant.
- is the temperature.
- is the intermolecular pair potential.
In other words, the Mayer function is the Boltzmann factor of the interaction potential, minus one.
Diagrammatically the Mayer f-function is written as
Hard sphere model[edit]
For the hard sphere model the Mayer f-function becomes:
where is the hard sphere diameter.
References[edit]
- Joseph Edward Mayer and Maria Goeppert Mayer "Statistical Mechanics" John Wiley and Sons (1940)
- Joseph E. Mayer "Contribution to Statistical Mechanics", Journal of Chemical Physics 10 pp. 629-643 (1942)