Ice Ih: Difference between revisions
Jump to navigation
Jump to search
m (Added an entropy subsection.) |
m (Moved location of a reference.) |
||
| Line 49: | Line 49: | ||
==Entropy== | ==Entropy== | ||
:''Main article: [[Entropy of ice phases]]'' | :''Main article: [[Entropy of ice phases]]'' | ||
*[http://dx.doi.org/10.1021/ja01315a102 Linus Pauling "The Structure and Entropy of Ice and of Other Crystals with Some Randomness of Atomic Arrangement", Journal of the American Chemical Society '''57''' pp. 2674 - 2680 (1935)] | |||
==Experimental data== | ==Experimental data== | ||
*[http://dx.doi.org/10.1063/1.2183324 Rainer Feistel and Wolfgang Wagner "A New Equation of State for H2O Ice Ih", Journal of Physical and Chemical Reference Data '''35''' pp. 1021- (2006)] | *[http://dx.doi.org/10.1063/1.2183324 Rainer Feistel and Wolfgang Wagner "A New Equation of State for H2O Ice Ih", Journal of Physical and Chemical Reference Data '''35''' pp. 1021- (2006)] | ||
| Line 54: | Line 55: | ||
<references/> | <references/> | ||
'''Related reading''' | '''Related reading''' | ||
*[http://dx.doi.org/10.1021/jp0743121 E. G. Noya, C. Menduiña, J. L. Aragones, and C. Vega "Equation of State, Thermal Expansion Coefficient, and Isothermal Compressibility for Ices Ih, II, III, V, and VI, as Obtained from Computer Simulation", Journal of Physical Chemistry C '''111''' pp. 15877 - 15888 (2007)] | *[http://dx.doi.org/10.1021/jp0743121 E. G. Noya, C. Menduiña, J. L. Aragones, and C. Vega "Equation of State, Thermal Expansion Coefficient, and Isothermal Compressibility for Ices Ih, II, III, V, and VI, as Obtained from Computer Simulation", Journal of Physical Chemistry C '''111''' pp. 15877 - 15888 (2007)] | ||
{{numeric}} | {{numeric}} | ||
[[category: water]] | [[category: water]] | ||
Revision as of 13:03, 17 July 2009
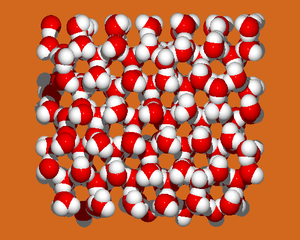
Ice Ih (hexagonal ice) is a proton disordered ice phase having the space group P63/mmc. Ice Ih has the following lattice parameters at 250 K: a=4.51842 Å, , and c=7.35556 Å with four molecules per unit cell (in Table 3 of [1]). The proton ordered form of ice Ih is known as ice XI, which (in principle) forms when ice Ih is cooled to below 72K (it is usually doped with KOH to aid the transition).
Melting point
The following is a collection of melting points for the ice Ih-water transition:
Pressure Water model/technique Reference 1 bar TIP3P [2] 1 bar SPC [2] 1 bar SPC/E / free energy calculation [3] 1 bar TTM2.1-F (quantum) [4] 1 bar TIP4P / free energy calculation [3] 1 bar TTM2.1-F (classical) [4] 1 bar TIP4P/Ew / free energy calculation [3] 1 bar TIP4P/2005 / free energy calculation [3] 1 bar TIP4P/Ice / free energy calculation [3] 1 bar experimental value 1 bar TIP5P [2] 1 bar NvdE [5] 10,000 bar Becke-Lee-Yang-Parr functional [6] 2500 bar Perdew-Burke-Ernzerhof functional [6]
Related reading
Radial distribution function
Phonon density of states
In [7] the phonon density of states for the POL1, TIPS2, TIP4P, TIP3P, SPC, Rowlinson, MCY, and BF models for water are compared to experiment.
Entropy
- Main article: Entropy of ice phases
Experimental data
References
- ↑ K. Röttger, A. Endriss, J. Ihringer, S. Doyle and W. F. Kuhs "Lattice constants and thermal expansion of H2O and D2O ice Ih between 10 and 265 K", Acta Crystallographica Section B 50 pp. 644-648 (1994)
- ↑ 2.0 2.1 2.2 C. Vega, J. L. F. Abascal, M. M. Conde and J. L. Aragones "What ice can teach us about water interactions: a critical comparison of the performance of different water models", Faraday Discussions 141 pp. 251-276 (2009)
- ↑ 3.0 3.1 3.2 3.3 3.4 Carlos Vega, Maria Martin-Conde and Andrzej Patrykiejew "Absence of superheating for ice Ih with a free surface: a new method of determining the melting point of different water models", Molecular Physics 104 pp. 3583-3592 (2006)
- ↑ 4.0 4.1 Francesco Paesani and Gregory A. Voth "Quantum Effects Strongly Influence the Surface Premelting of Ice", Journal of Physical Chemistry C 112 pp. 324-327 (2008)
- ↑ José L. F. Abascal, Ramón García Fernández, Carlos Vega and Marcelo A. Carignano, "The melting temperature of the six site potential model of water", Journal of Chemical Physics, 125 166101 (2006)
- ↑ 6.0 6.1 Soohaeng Yoo, Xiao Cheng Zeng, and Sotiris S. Xantheas "On the phase diagram of water with density functional theory potentials: The melting temperature of ice Ih with the Perdew–Burke–Ernzerhof and Becke–Lee–Yang–Parr functionals", Journal of Chemical Physics 130 221102 (2009)
- ↑ Shunle Dong and Jichen Li "The test of water potentials by simulating the vibrational dynamics of ice", Physica B 276-278 pp. 469-470 (2000)
Related reading
















