Critical points: Difference between revisions
Carl McBride (talk | contribs) (Started tidy up.) |
Carl McBride (talk | contribs) m (Added a reference.) |
||
| Line 1: | Line 1: | ||
[[Image:press_temp.png|thumb|right]] | [[Image:press_temp.png|thumb|right]] | ||
The '''critical point''' is a point found at the end of the liquid-vapour coexistence curve (the red point shown on the [[pressure-temperature]] plot on the right). At this point the [[temperature]] is known as the ''critical temperature'' <math>(T_c)</math> | The '''critical point''', discovered in 1822 by Charles Cagniard de la Tour <ref>Charles Cagniard de la Tour "", Annales de chimie et de physique '''21''' pp. 127- (1822)</ref>, is a point found at the end of the liquid-vapour coexistence curve (the red point shown on the [[pressure-temperature]] plot on the right). At this point the [[temperature]] is known as the ''critical temperature'' <math>(T_c)</math> | ||
and the [[pressure]] is known as the ''critical pressure'' <math>(P_c)</math>. | and the [[pressure]] is known as the ''critical pressure'' <math>(P_c)</math>. | ||
For an interesting discourse on the "discovery" of the liquid-vapour critical point, the Bakerian Lecture of [[Thomas Andrews]] | For an interesting discourse on the "discovery" of the liquid-vapour critical point, the Bakerian Lecture of [[Thomas Andrews]] | ||
Revision as of 17:01, 20 November 2009
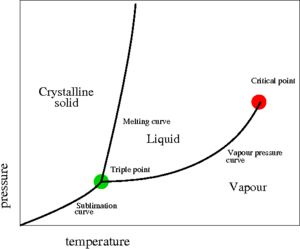
The critical point, discovered in 1822 by Charles Cagniard de la Tour [1], is a point found at the end of the liquid-vapour coexistence curve (the red point shown on the pressure-temperature plot on the right). At this point the temperature is known as the critical temperature and the pressure is known as the critical pressure . For an interesting discourse on the "discovery" of the liquid-vapour critical point, the Bakerian Lecture of Thomas Andrews makes good reading [2]. Critical points are singularities in the partition function. In the critical point vicinity (Ref. [3] Eq. 17a)
and
For a review of the critical region see the work of Michael E. Fisher [4]
"... Turning now to the question of specific heats, it has long been known that real gases exhibit a large ``anomalous" specific-heat maximum above which lies near the critical isochore and which is not expected on classical theory..."
also
"... measurements (Ref. [5] ) of for argon along the critical isochore suggest strongly that . Such a result is again inconsistent with classical theory."
Thus in the vicinity of the liquid-vapour critical point, both the isothermal compressibility and the heat capacity at constant pressure diverge to infinity.
Liquid-liquid critical point
Solid-liquid critical point
It is widely held that there is no solid-liquid critical point. The reasoning behind this was given on the grounds of symmetry by Landau and Lifshitz [6]. However, recent work using the Z2 potential suggests that this may not be the last word on the subject. [7].
Tricritical points
- Robert B. Griffiths "Thermodynamics Near the Two-Fluid Critical Mixing Point in He3 - He4", Physical Review Letters 24 715-717 (1970)
- Lech Longa "On the tricritical point of the nematic–smectic A phase transition in liquid crystals", Journal of Chemical Physics 85 pp. 2974-2985 (1986)
Critical exponents
- Main article: Critical exponents
See also
References
- ↑ Charles Cagniard de la Tour "", Annales de chimie et de physique 21 pp. 127- (1822)
- ↑ Thomas Andrews "The Bakerian Lecture: On the Continuity of the Gaseous and Liquid States of Matter", Philosophical Transactions of the Royal Society of London 159 pp. 575-590 (1869)
- ↑ G. A. Martynov; G. N. Sarkisov "Exact equations and the theory of liquids. V", Molecular Physics 49 pp. 1495-1504 (1983)
- ↑ Michael E. Fisher "Correlation Functions and the Critical Region of Simple Fluids", Journal of Mathematical Physics 5 pp. 944-962 (1964)
- ↑ A. Michels, J.M. Levelt and G.J. Wolkers "Thermodynamic properties of argon at temperatures between 0°C and −140°C and at densities up to 640 amagat (pressures up to 1050 atm.)", Physica 24 pp. 769-794 (1958)
- ↑ L. D. Landau and E. M. Lifshitz, "Statistical Physics" (Course of Theoretical Physics, Volume 5) 3rd Edition Part 1, Chapter XIV, Pergamon Press (1980) § 83 p. 258
- ↑ Måns Elenius and Mikhail Dzugutov "Evidence for a liquid-solid critical point in a simple monatomic system", Journal of Chemical Physics 131, 104502 (2009)
Related reading
- M. I. Bagatskii and A. V. Voronel and B. G. Gusak "", Journal of Experimental and Theoretical Physics 16 pp. 517- (1963)
- Robert B. Griffiths and John C. Wheeler "Critical Points in Multicomponent Systems", Physical Review A 2 1047 - 1064 (1970)
- Michael E. Fisher "The renormalization group in the theory of critical behavior", Reviews of Modern Physics 46 pp. 597 - 616 (1974)
- J. V. Sengers and J. M. H. Levelt Sengers "Thermodynamic Behavior of Fluids Near the Critical Point", Annual Review of Physical Chemistry 37 pp. 189-222 (1986)
- Kamakshi Jagannathan and Arun Yethiraj "Molecular Dynamics Simulations of a Fluid near Its Critical Point", Physical Review Letters 93 015701 (2004)
- Cyril Domb "The Critical Point: A Historical Introduction To The Modern Theory Of Critical Phenomena", Taylor and Francis (1996) ISBN 9780748404353






