Argon: Difference between revisions
Jump to navigation
Jump to search
Carl McBride (talk | contribs) m (Placed numeric template at the end of the article.) |
Carl McBride (talk | contribs) m (Added a classic reference.) |
||
| Line 1: | Line 1: | ||
[[Image:Lennard-Jones.png|thumb| The Lennard-Jones model for argon.]] | [[Image:Lennard-Jones.png|thumb| The Lennard-Jones model for argon.]] | ||
'''Argon''' has a mass of 39.948 [[atomic mass units | umas]]. Sadus and Prausnitz have shown that three-body repulsion makes a significant contribution to [[Idealised models#Three-body potentials|three-body interactions]] in the liquid phase (Ref. | '''Argon''' has a mass of 39.948 [[atomic mass units | umas]]. Sadus and Prausnitz have shown that three-body repulsion makes a significant contribution to [[Idealised models#Three-body potentials|three-body interactions]] in the liquid phase (Ref. 5) (for use of the [[Axilrod-Teller interaction]] see Refs. 6 and 7). However, the [[Lennard-Jones model]] has been frequently used due to its simplicity. | ||
==Lennard-Jones parameters== | ==Lennard-Jones parameters== | ||
The [[Lennard-Jones model |Lennard-Jones]] parameters for liquid argon are listed in the following table: | The [[Lennard-Jones model |Lennard-Jones]] parameters for liquid argon are listed in the following table: | ||
{| border="1" | {| border="1" | ||
|- | |- | ||
| Authors || <math>\epsilon/k_B</math> (K) || <math>\sigma</math> (nm)|| | | Authors || <math>\epsilon/k_B</math> (K) || <math>\sigma</math> (nm)|| Reference | ||
|- | |- | ||
| Rowley, Nicholson and Parsonage || 119.8 || 0.3405 || Ref. | | Rowley, Nicholson and Parsonage || 119.8 || 0.3405 || Ref. 8 | ||
|- | |- | ||
|Barker, Fisher and Watts ||142.095 || 0.33605 || Ref. | |Barker, Fisher and Watts ||142.095 || 0.33605 || Ref. 9 | ||
|- | |- | ||
| White || 125.7 || 0.3345 || Ref. | | White || 125.7 || 0.3345 || Ref. 10 parameter set #4 | ||
|} | |} | ||
==References== | ==References== | ||
#[http://dx.doi.org/10.1080/00268976400100611 H. C. Longuet-Higgins and B. Widom "A rigid sphere model for the melting of argon", Molecular Physics '''8''' pp. 549-556 (1964)] | #[http://dx.doi.org/10.1080/00268976400100611 H. C. Longuet-Higgins and B. Widom "A rigid sphere model for the melting of argon", Molecular Physics '''8''' pp. 549-556 (1964)] | ||
#[http://dx.doi.org/10.1080/00268976800100721 D. Henderson and J. A. Barker "On the solidification of argon", Molecular Physics '''14''' pp. 587-589 (1968)] | #[http://dx.doi.org/10.1080/00268976800100721 D. Henderson and J. A. Barker "On the solidification of argon", Molecular Physics '''14''' pp. 587-589 (1968)] | ||
#[http://dx.doi.org/10.1080/00268977100101821 J. A. Barker, R. A. Fisher and R. O. Watts "Liquid argon: Monte Carlo and molecular dynamics calculations", Molecular Physics '''21''' pp. 657-673 (1971)] | |||
#[http://dx.doi.org/10.1103/PhysRevA.5.2238 F. Lado "Numerical Calculation of the Density Autocorrelation Function for Liquid Argon", Physical Review A '''5''' pp. 2238-2244 (1972)] | #[http://dx.doi.org/10.1103/PhysRevA.5.2238 F. Lado "Numerical Calculation of the Density Autocorrelation Function for Liquid Argon", Physical Review A '''5''' pp. 2238-2244 (1972)] | ||
#[http://dx.doi.org/10.1063/1.471172 Richard J. Sadus and J. M. Prausnitz "Three-body interactions in fluids from molecular simulation: Vapor–liquid phase coexistence of argon", Journal of Chemical Physics '''104''' pp. 4784-4787 (1996)] | #[http://dx.doi.org/10.1063/1.471172 Richard J. Sadus and J. M. Prausnitz "Three-body interactions in fluids from molecular simulation: Vapor–liquid phase coexistence of argon", Journal of Chemical Physics '''104''' pp. 4784-4787 (1996)] | ||
Revision as of 11:50, 5 August 2008
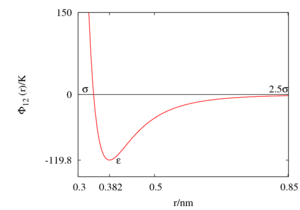
Argon has a mass of 39.948 umas. Sadus and Prausnitz have shown that three-body repulsion makes a significant contribution to three-body interactions in the liquid phase (Ref. 5) (for use of the Axilrod-Teller interaction see Refs. 6 and 7). However, the Lennard-Jones model has been frequently used due to its simplicity.
Lennard-Jones parameters
The Lennard-Jones parameters for liquid argon are listed in the following table:
| Authors | (K) | (nm) | Reference |
| Rowley, Nicholson and Parsonage | 119.8 | 0.3405 | Ref. 8 |
| Barker, Fisher and Watts | 142.095 | 0.33605 | Ref. 9 |
| White | 125.7 | 0.3345 | Ref. 10 parameter set #4 |
References
- H. C. Longuet-Higgins and B. Widom "A rigid sphere model for the melting of argon", Molecular Physics 8 pp. 549-556 (1964)
- D. Henderson and J. A. Barker "On the solidification of argon", Molecular Physics 14 pp. 587-589 (1968)
- J. A. Barker, R. A. Fisher and R. O. Watts "Liquid argon: Monte Carlo and molecular dynamics calculations", Molecular Physics 21 pp. 657-673 (1971)
- F. Lado "Numerical Calculation of the Density Autocorrelation Function for Liquid Argon", Physical Review A 5 pp. 2238-2244 (1972)
- Richard J. Sadus and J. M. Prausnitz "Three-body interactions in fluids from molecular simulation: Vapor–liquid phase coexistence of argon", Journal of Chemical Physics 104 pp. 4784-4787 (1996)
- Phil Attard "Pair-hypernetted-chain closure for three-body potentials: Results for argon with the Axilrod-Teller triple-dipole potential", Physical Review A 45 pp. 3659-3669 (1992)
- J. A. Anta, E. Lomba and M. Lombardero "Influence of three-body forces on the gas-liquid coexistence of simple fluids: The phase equilibrium of argon", Physical Review E 55 pp. 2707-2712 (1997)
- L. A. Rowley, D. Nicholson and N. G. Parsonage "Monte Carlo grand canonical ensemble calculation in a gas-liquid transition region for 12-6 Argon", Journal of Computational Physics 17 pp. 401-414 (1975)
- J. A. Barker, R. A. Fisher and R. O. Watts "Liquid argon: Monte carlo and molecular dynamics calculations", Molecular Physics 21 pp. 657-673 (1971)
- John A. White "Lennard-Jones as a model for argon and test of extended renormalization group calculations", Journal of Chemical Physics 111 pp. 9352-9356 (1999)

