Argon: Difference between revisions
Carl McBride (talk | contribs) m (Started a tidy of the introduction) |
Carl McBride (talk | contribs) (Added the historical Wood and Parker paper) |
||
| Line 1: | Line 1: | ||
'''Argon''' (Ar) has long been a popular choice for [[Computer simulation techniques |computer simulations]] of simple liquids. | '''Argon''' (Ar) has long been a popular choice for [[Computer simulation techniques |computer simulations]] of simple liquids. Some of the first computer simulations of liquid argon were those of Wood and Parker in 1957 <ref>[http://dx.doi.org/10.1063/1.1743822 W. W. Wood and F. R. Parker "Monte Carlo Equation of State of Molecules Interacting with the Lennard‐Jones Potential. I. A Supercritical Isotherm at about Twice the Critical Temperature", Journal of Chemical Physics '''27''' pp. 720- (1957)]</ref> and of Rahman in 1964 <ref name="Rahman" >[http://dx.doi.org/10.1103/PhysRev.136.A405 A. Rahman "Correlations in the Motion of Atoms in Liquid Argon", Physical Review '''136''' pp. A405–A411 (1964)]</ref>. Sadus and Prausnitz have shown that three-body repulsion makes a significant contribution to [[Idealised models#Three-body potentials|three-body interactions]] in the liquid phase | ||
<ref>[http://dx.doi.org/10.1063/1.471172 Richard J. Sadus and J. M. Prausnitz "Three-body interactions in fluids from molecular simulation: Vapor–liquid phase coexistence of argon", Journal of Chemical Physics '''104''' pp. 4784-4787 (1996)]</ref> | <ref>[http://dx.doi.org/10.1063/1.471172 Richard J. Sadus and J. M. Prausnitz "Three-body interactions in fluids from molecular simulation: Vapor–liquid phase coexistence of argon", Journal of Chemical Physics '''104''' pp. 4784-4787 (1996)]</ref> | ||
(for use of the [[Axilrod-Teller interaction]] see | (for use of the [[Axilrod-Teller interaction]] see | ||
Revision as of 16:53, 9 June 2011
Argon (Ar) has long been a popular choice for computer simulations of simple liquids. Some of the first computer simulations of liquid argon were those of Wood and Parker in 1957 [1] and of Rahman in 1964 [2]. Sadus and Prausnitz have shown that three-body repulsion makes a significant contribution to three-body interactions in the liquid phase [3] (for use of the Axilrod-Teller interaction see [4] [5]). However, the generic Lennard-Jones model has been frequently used due to its simplicity; some parameters are quoted in the next section. A specific interatomic potential for Ar has been proposed by Aziz [6].
Thermophysical properties (experimental)
| Property [7] | Temperature | Pressure |
| Triple point | 83.8058 K | 69 kPa |
| Critical point | 150.87 K | 4.898 MPa |
| Melting point | 83.80 K | |
| Boiling point | 87.30 K |
Lennard-Jones parameters
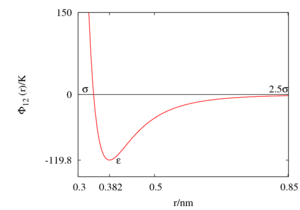
A selection of parameters for the Lennard-Jones model for liquid argon are listed in the following table:
| Authors | (K) | (nm) | Reference (year) |
| Rahman | 120 | 0.34 | [2] (1964) |
| Barker, Fisher and Watts | 142.095 | 0.33605 | [8] (1971) |
| Rowley, Nicholson and Parsonage | 119.8 | 0.3405 | [9] (1975) |
| White | 125.7 | 0.3345 | [10] parameter set #4 (1999) |
Buckingham potential
The Buckingham potential for argon is given by (Eq. 27 [11]):
where is in ergs ( 10−7 J) and in Å.
BBMS potential
The Bobetic-Barker-Maitland-Smith potential [12] [13].
Radial distribution function
Nucleation
Quantum simulations
References
- ↑ W. W. Wood and F. R. Parker "Monte Carlo Equation of State of Molecules Interacting with the Lennard‐Jones Potential. I. A Supercritical Isotherm at about Twice the Critical Temperature", Journal of Chemical Physics 27 pp. 720- (1957)
- ↑ 2.0 2.1 A. Rahman "Correlations in the Motion of Atoms in Liquid Argon", Physical Review 136 pp. A405–A411 (1964)
- ↑ Richard J. Sadus and J. M. Prausnitz "Three-body interactions in fluids from molecular simulation: Vapor–liquid phase coexistence of argon", Journal of Chemical Physics 104 pp. 4784-4787 (1996)
- ↑ Phil Attard "Pair-hypernetted-chain closure for three-body potentials: Results for argon with the Axilrod-Teller triple-dipole potential", Physical Review A 45 pp. 3659-3669 (1992)
- ↑ J. A. Anta, E. Lomba and M. Lombardero "Influence of three-body forces on the gas-liquid coexistence of simple fluids: The phase equilibrium of argon", Physical Review E 55 pp. 2707-2712 (1997)
- ↑ Ronald A. Aziz "A highly accurate interatomic potential for argon", Journal of Chemical Physics 99 p. 4518 (1993)
- ↑ Physical properties of Argon on webelements
- ↑ J. A. Barker, R. A. Fisher and R. O. Watts "Liquid argon: Monte carlo and molecular dynamics calculations", Molecular Physics 21 pp. 657-673 (1971)
- ↑ L. A. Rowley, D. Nicholson and N. G. Parsonage "Monte Carlo grand canonical ensemble calculation in a gas-liquid transition region for 12-6 Argon", Journal of Computational Physics 17 pp. 401-414 (1975)
- ↑ John A. White "Lennard-Jones as a model for argon and test of extended renormalization group calculations", Journal of Chemical Physics 111 pp. 9352-9356 (1999)
- ↑ R. A. Buckingham "The Classical Equation of State of Gaseous Helium, Neon and Argon", Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences 168 pp. 264-283 (1938)
- ↑ M. V. Bobetic and J. A. Barker "Lattice Dynamics with Three-Body Forces: Argon", Physical Review B 2 4169-4175 (1970)
- ↑ G. C. Maitland and E. B. Smith "The intermolecular pair potential of argon", Molecular Physics 22 pp. 861-868 (1971)
Related material
- H. C. Longuet-Higgins and B. Widom "A rigid sphere model for the melting of argon", Molecular Physics 8 pp. 549-556 (1964)
- D. Henderson and J. A. Barker "On the solidification of argon", Molecular Physics 14 pp. 587-589 (1968)
- F. Lado "Numerical Calculation of the Density Autocorrelation Function for Liquid Argon", Physical Review A 5 pp. 2238-2244 (1972)
- Ali Asghar Davoodi and Farzaneh Feyzi "A new approach for long range corrections in molecular dynamics simulation with application to calculation of argon properties", Journal of Molecular Liquids 150 pp. 33-38 (2009)




